Differences In Administration Efficacy And Conditions Treated
Robert Burakoff, MD, MPH, is board-certified in gastroentrology. He is the vice chair for ambulatory services for the department of medicine at Weill Cornell Medical College in New York, where he is also a professor. He was the founding editor and co-editor in chief of Inflammatory Bowel Diseases.
Biologics are a newer class of drugs used to treat the inflammation that is caused by inflammatory bowel disease . This is a broad range of medications that each work in slightly different ways with different standards of administration and dosing. Some are approved to treat just one form of IBD, while others are used to treat both Crohn’s disease and ulcerative colitis.
Because biologic drugs temper the immune response, people taking them are prone to certain infections. It’s important, therefore, to take steps to reduce your vulnerability. People with IBD should receive vaccinations, ideally before starting a biologic, although many immunizations can also be given while taking a biologic.
According to 2020 guidelines, a biologic drug should be used first-line for treatment in people with moderate to severe ulcerative colitis.
Infusions Every 8 Weeks After 3 Starter Doses
REMICADE® is given as an intravenous infusion by a healthcare professional through a needle placed in a vein in your arm.
Given over a period of about 2 hours
Weeks 0, 2, and 6
After starter doses, 1 maintenance dose is infused every 8 weeks
Your doctor will determine the right dosage of REMICADE® for you.
Key Points About Simponi
- Simponi is approved to treat ulcerative colitis.
- Simponi is given by injection at home.
- Simponi is started with two injections, followed by one injection two weeks later, and one injection every four weeks thereafter.
- Common side effects include pain or irritation at the injection site and upper respiratory or viral infections.
- If you are pregnant or plan to become pregnant, you and your doctor should decide if you should take Simponi.
- It’s not currently known how Simponi will affect a nursing infant.
- Simponi must be refrigerated.
Also Check: Probiotics Good For Ulcerative Colitis
Alternatives To Humira For Ulcerative Colitis
- 20mg/0.4mL
Indicated for reduction of signs and symptoms of moderately-to-severely active polyarticular juvenile idiopathic arthritis
May be administered with methotrexate, glucocorticoids, nonsteroidal anti-inflammatory drugs , or analgesics
Humira
- < 2 years or < 10 kg: Safety and efficacy not established
-
â¥2 years
- 15 kg to < 30 kg: 20 mg SC q2wk
- â¥30 kg: 40 mg SC q2wk
Cyltezo
- < 2 years or < 10 kg: Safety and efficacy not established
- < 15 kg: No dosage form available for weight-based dosing
-
â¥2 years
- 15 to < 30 kg: 20 mg SC q2wk
- â¥30 kg: 40 mg SC q2wk
Yusimry
- â¥2 years and â¥30 kg: 40 mg SC q2wk
Amjevita, Hadlima, Hyrimoz
- < 4 years: Safety and efficacy not established
-
â¥4 years
- Amjevita, Hulio: 15 to < 30 kg: 20 mg SC q2wk
- Amjevita, Hadlima, Hulio, or Hyrimoz: â¥30 kg: 40 mg SC q2wk
Receiving Remicade With Other Drugs
If you have RA, your doctor can only prescribe Remicade with methotrexate.
Flare-ups of inflammatory diseases may require taking corticosteroids.
People using Remicade with methotrexate or corticosteroids have a higher risk for developing a serious infection. This is because Remicade, methotrexate, and corticosteroids are all immunosuppressants. They reduce the activity of the immune system, decreasing the ability of your body to fight infections.
Other medications you may need to take with Remicade include:
- aminosalicylates, including mesalamine and sulfasalazine
Don’t Miss: Can Diet Help Ulcerative Colitis
What Is Remicade And Humira
Infliximab and Adalimumab belongs to a new class of drugs known as monoclonal antibodies. These particular drugs blocks the activity of an inflammatory chemical in the tissue called tumor necrosis factor . Excessive TNF seems to lead to increased inflammation and damage in the tissues in disorders like Crohns disease and rheumatoid arthritis. Because Remicade/Humira block TNF, they are known as anti-TNF.
Could Remicade Stop Working For Me
Yes. Remicade may become less effective for you over time. This can occur if your bodys immune system starts to recognize Remicade as a foreign invader and makes antibodies to Remicade. Antibodies are immune system proteins that fight foreign substances, including medications such as Remicade.
If your body makes anti-Remicade antibodies, the drug will clear from your system faster and wont be as effective.
Remicade may also stop working for a time because of stress, dietary choices, or other health conditions.
If youre concerned about how effective Remicade is in treating your condition, talk with your doctor.
Read Also: How To Stop Ulcerative Colitis Pain
What Should I Tell My Doctor Before Starting Humira
Tell your doctor about all of your health conditions, including if you:
- Have an infection, are being treated for infection, or have symptoms of an infection
- Get a lot of infections or infections that keep coming back
- Have diabetes
- Have TB or have been in close contact with someone with TB, or were born in, lived in, or traveled where there is more risk for getting TB
- Live or have lived in an area where there is an increased risk for getting certain kinds of fungal infections, such as histoplasmosis, coccidioidomycosis, or blastomycosis. These infections may happen or become more severe if you use HUMIRA. Ask your doctor if you are unsure if you have lived in these areas
- Have or have had hepatitis B
- Are scheduled for major surgery
- Have or have had cancer
- Have numbness or tingling or a nervous system disease such as multiple sclerosis or Guillain-Barré syndrome
- Have or had heart failure
- Have recently received or are scheduled to receive a vaccine. HUMIRA patients may receive vaccines, except for live vaccines. Children should be brought up to date on all vaccines before starting HUMIRA
- Are allergic to rubber, latex, or any HUMIRA ingredients
- Are pregnant, planning to become pregnant, breastfeeding, or planning to breastfeed
- Have a baby and you were using HUMIRA during your pregnancy. Tell your babys doctor before your baby receives any vaccines
Is It Safe To Have The Vaccine If I’m Pregnant Or Planning To Become Pregnant
You and your developing baby cannot catch COVID-19 from having the vaccine. This is because the coronavirus vaccine is a non-live vaccine. This means the virus cannot grow in your body and cause an infection. Other non-live vaccines are already routinely offered during pregnancy. These include the whooping cough and flu vaccines. There have been no proven risks or long-term effects to the mother or baby from these vaccines. There is also no evidence to suggest the coronavirus vaccine would affect female or male fertility.
You can have the COVID-19 vaccine if:
- you’re pregnant or think you might be
- you’re breastfeeding
The NHS has more information on Pregnancy, breastfeeding, fertility and COVID-19 vaccination.
To help you make the right decision for you, the RCOG have made a helpful guide for pregnant women.
Also Check: Is Alcohol Bad For Ulcerative Colitis
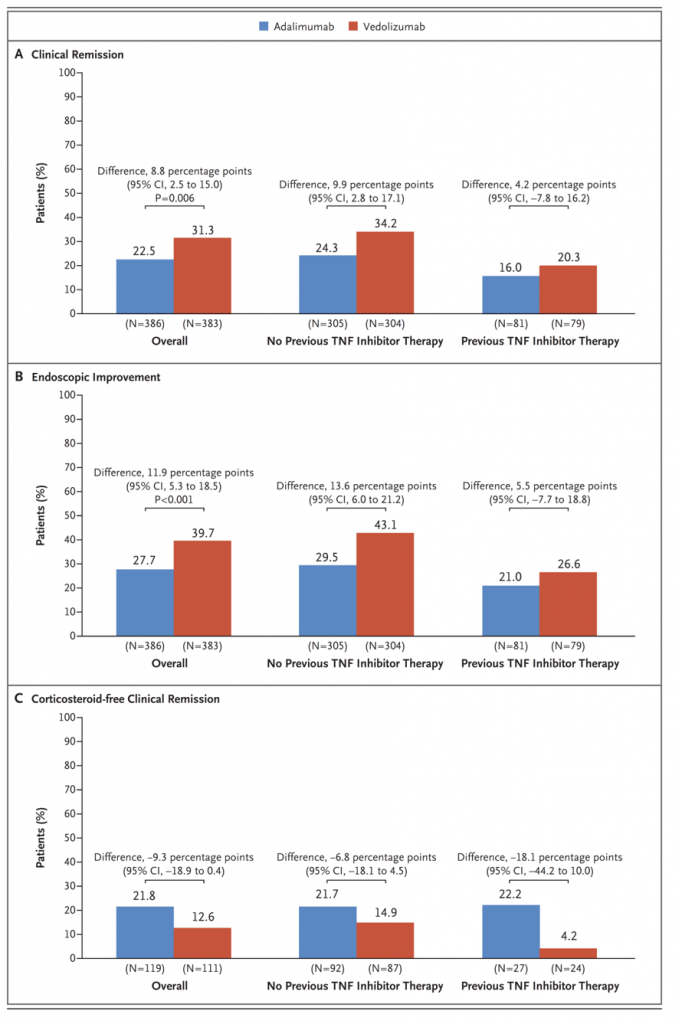
Management Of Ulcerative Colitis
The main treatment goals for ulcerative colitis are the induction and maintenance of clinical and endoscopic remission. As far as mild-to-moderate disease is concerned, the oral and topical aminosalicylates represent the standard therapy for achieving this outcome. In the event of inadequate response to aminosalicylates and in patients with moderate-to-severe disease, systemic corticosteroids are the best option for inducing remission. Patients with active ulcerative colitis who do not have significant clinical improvement after 24 weeks of an appropriate course of corticosteroids are classified as corticosteroid-refractory. Anti-tumor necrosis factor-alpha monoclonal antibodies represent the best available option for this group of patients, achieving clinical and endoscopic remission without prolonged steroid exposure.
Patients with acute, severe ulcerative colitis need to be hospitalized and treated with intensive intravenous corticosteroids . A lack of improvement within 35 days of intensive treatment is an indication for rescue therapy or surgery. A recent open-label trial involving 115 patients with acute, severe ulcerative colitis who were refractory to intravenous corticosteroids and randomized to receive either intravenous cyclosporine or infliximab has shown no significant differences in treatment failure .
Adalimumab In Ulcerative Colitis
Adalimumab is a human monoclonal immunoglobulin G1 antibody to TNF that is subcutaneously administered at a standard induction dose of 160 mg, followed by 80 mg after 2 weeks. Maintenance doses are then scheduled at 40 mg every other week . This drug has been shown to be effective for inducing and maintaining remission in patients with active, moderate-to-severe luminal or perianal Crohns disease patients naïve to anti-TNF or patients with previous loss of response or intolerance to infliximab.
As far as ulcerative colitis is concerned after the publication of the results of the two pivotal, randomized placebo-controlled double-blind trials ,, adalimumab was approved for use in patients with moderate-to-severe active disease and in those who were nonresponders or intolerant to conventional therapy. In these trials, involving more than 1000 patients with moderate-to-severe active ulcerative colitis, adalimumab was compared with placebo with regard to the efficacy of induction and as a maintenance treatment, assessed after 8 and 52 weeks, respectively.
Recommended Reading: How Serious Is A Stomach Ulcer
Patients Having Received First
Baseline characteristics
Infliximab was prescribed as first-line anti-TNF treatment in 126 patients , whereas adalimumab was prescribed in 34 patients . The median time interval between disease onset and the initiation of maintenance treatment with infliximab or adalimumab as first-line anti-TNF treatment was 3 years.
The medications taken before the initiation of an anti-TNF agent were variously 5-aminosalicylate , systemic steroids , azathioprine , methotrexate , rectal therapy , cyclosporine , enteral or parenteral nutrition , and investigational drugs .
A total of 130 patients were receiving other treatments on initiation of first-line treatment with an anti-TNF agent: 60 patients were receiving 5-aminosalicylate, 88 patients were receiving systemic steroids, 57 patients were receiving azathioprine, 11 patients were receiving methotrexate, 10 patients were receiving rectal therapy, and 3 patients were receiving enteral or parenteral nutrition.
Detailed data on all treatments before or on initiation of first-line treatment with an anti-TNF agent are given in Supplementary Table S1.
Retention rates and reasons for anti-TNF withdrawal
Persistence of first-line treatment with an anti-TNF agent
The overall mean persistence of first-line treatment with an anti-TNF agent was 3.1 years, with values of 3.4 years for infliximab and 2.1 years for adalimumab. The difference in persistence between the infliximab and adalimumab subgroups was not statistically significant .
How Should Remicade Be Taken
- You will be given Remicade through a needle placed in a vein in your arm.
- Your doctor may decide to give you medicine before starting the Remicade infusion to prevent or lessen side effects.
- Only a healthcare professional should prepare the medicine and administer it to you.
- Remicade will be given to you over a period of about 2 hours.
If you have side effects from Remicade, the infusion may need to be adjusted or stopped. In addition, your healthcare professional may decide to treat your symptoms.
A healthcare professional will monitor you during the Remicade infusion and for a period of time afterward for side effects. Your doctor may do certain tests while you are taking Remicade to monitor you for side effects and to see how well you respond to the treatment.
Your doctor will determine the right dose of Remicade for you and how often you should receive it. Make sure to discuss with your doctor when you will receive infusions and to come in for all your infusions and follow-up appointments.
You May Like: Stomach Ulcer Blood In Stool
Can I Switch Between Stelara And Humira
The short answer: Its possible.
Details: Stelara and Humira are both biologic disease-modifying antirheumatic drugs . Biologics are newer, targeted therapies derived from living sources. And DMARDs suppress certain parts of your immune system to help reduce inflammation . This helps prevent damage to your healthy tissues and symptoms of your condition.
These drugs are both used to treat similar autoimmune or inflammatory conditions, such as psoriatic arthritis or ulcerative colitis . So you may be able to switch between Stelara and Humira.
In some cases, your doctor may recommend the switch. For example:
- If you have psoriatic arthritis thats not controlled with Stelara, your doctor may suggest switching to Humira. This switch is recommended by the American College of Rheumatology.
- According to American Gastroenterological Association treatment guidelines, Stelara and Humira are both first-choice treatments for moderate to severe ulcerative colitis. So if you need to switch because of side effects or costs, it may be possible.
However, choosing to switch between these drugs isnt always a simple decision. Your doctor may or may not recommend it based on many factors, such as:
- your condition and other medical treatments
- your experience with past treatments
- your risk for serious side effects
- any side effects youve had
- your other medications
- recommendations from the latest treatment guidelines
- drug costs or availability
Stelara And Other Medications Or Therapies
Below are lists of medications and therapies that can interact with Stelara. These lists do not contain all the drugs that may interact with Stelara.
Before taking Stelara, talk with your doctor and pharmacist. Tell them about all prescription, over-the-counter, and other drugs you take. Also tell them about any vitamins, herbs, and supplements you use. Sharing this information can help you avoid potential interactions.
If you have questions about drug interactions that may affect you, ask your doctor or pharmacist.
Stelara and vaccines
You shouldnt get a live vaccine when youre using Stelara. Getting a live vaccine during Stelara treatment increases your risk of getting the condition the vaccine is meant to prevent.
This is because Stelara suppresses your immune systems ability to fight infections. Receiving a live vaccine during Stelara treatment increases your risk of serious infections.
Examples of live vaccines that you should avoid during Stelara treatment include:
You should also avoid getting the Bacillus Calmette-Guérin vaccine for 1 year before you start using Stelara, during your Stelara treatment, and for 1 year after you stop using Stelara. The BCG vaccine is meant to prevent tuberculosis . Its more commonly given to people who live outside of the United States.
Stelara and allergy shots
Stelara and warfarin
If youre taking these drugs together, your doctor may need to adjust your dosage of either Stelara or warfarin.
- everolimus
Also Check: How Do You Get Ulcers In Your Stomach
Are Remicade And Humira Safe To Take During Pregnancy Or While Breastfeeding
Researchers haven’t conducted studies of either of these drugs in pregnant or nursing mothers, though monkey studies of Humira showed no ill effects to the fetuses.
In the absence of research, doctors must weigh the risks and benefits before prescribing Remicade or Humira to pregnant or breastfeeding mothers.
Because of the potential immune suppression effects, however, the prescribing information recommends against administering live vaccines to a baby within six months of being exposed to a TNF inhibitor.
Can I Have An Antibody Test After My Vaccine
Antibody testingforcoronavirusisnotyetwidely available for free on the NHS.This is because antibodytestscurrently cannotgive a clearindicationof how wellyou areprotected from COVID-19.
The British Society of Gastroenterology have stated that antibody testing is not currently recommended in routine clinical practice. Read the BSG position statement here.
Antibodies are proteins made by your body to fight infection.Anantibody test is a blood testthatlooks at antibodies tocoronavirus.The testresultmaytell you yourlevelof antibodies, or just a positive or negative result.
An antibody test can help to see if you have been exposed to the virus before whether that is from infection or a vaccination. However, it cannot tellyouyouroveralllevel of immunity.
This is for a few reasons.
Another issuewith antibody testing isthatit may give people a false sense of securityif their antibodylevelsarehigh. Or on the other hand,the test couldmake people think they are extremely high risk ifthe resultis negative or very low, even if theyactually havea good immune defence.
Therearea few groups that can get antibody tests for free.
- Those that are taking part in certain COVID-19 studies may be able to have antibody tests.
- Certain occupations can also make you eligible for antibody testing. Seegovernment advice for more information.
These results will not be able to tell you the likelihood of getting the virus in the future.
Don’t Miss: Primary Sclerosing Cholangitis Ulcerative Colitis
What Are Biologics For Ulcerative Colitis
Biologics are medications that doctors use to treat chronic inflammatory conditions such as inflammatory bowel disease . Ulcerative colitis is one type of IBD.
These laboratory-made antibodies are targeted to block specific proteins responsible for the inflammation that drives ulcerative colitis. This makes biologics different from medications such as corticosteroids, which may cause more severe side effects.
The Food and Drug Administration has approved the following biologics to treat moderate-to-severe ulcerative colitis:
- anti-tumor necrosis factor agents, including:
- infliximab
- adalimumab
- golimumab
The FDA approved the biosimilars of infliximab and adalimumab. As their name suggests, biosimilars are very similar to the originally approved biologics but may be more cost effective.
A person may receive biologics as an injection, as an infusion through an intravenous line, or by mouth.
The method of use, dosage, and frequency varies from one type of biologic to another.
Doctors typically prescribe an anti-TNF agent before they prescribe other types of biologics for moderate-to-severe ulcerative colitis. This is because anti-TNF medications are the most studied treatments.
Doctors may prescribe another type of biologic or a JAK inhibitor if the anti-TNF agent:
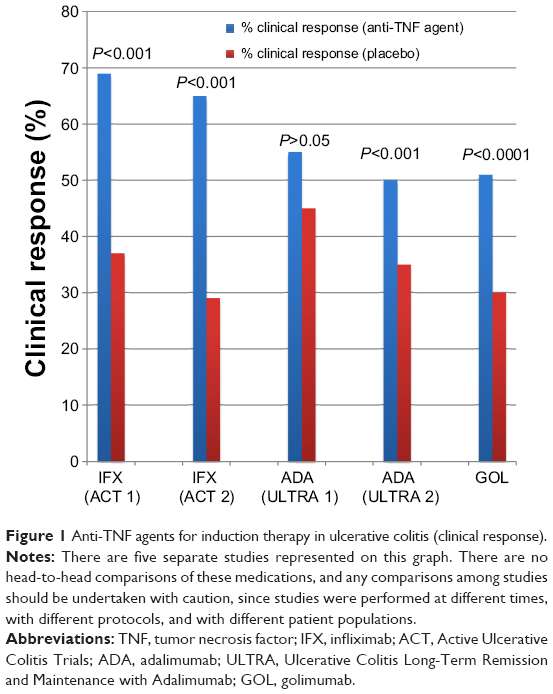
Rapid Remission & Response At Week 8
In two pivotal UC trials, patients treated with HUMIRA achieved rapid remission* at Week 8.
ULTRA 1: Remission for Total Population at Week 8 All Patients Were Bionaïve1
18.5%
P< 0.05
ULTRA 2: Remission for Total Population at Week 8 Patients Were Bionaïve and Bioexperienced1
16.5%
Limitation of Use: The effectiveness of HUMIRA has not been established in patients who have lost response to or were intolerant to TNF blockers.1
ULTRA 1 Study Design Intro: 8-week, randomized, double-blind, placebo-controlled study in 390anti-TNF-naïve adult patients with inadequate response to concurrent or prior immunosuppressant therapy and evaluated 3 treatment arms. Primary endpoint: percentage of patients achieving clinical remission at Week 8.1,2
ULTRA 2 Study Design Intro: 52-week, randomized, doubleblind, placebo-controlled study in 518 adult patients with inadequate response to concurrent or prior immunosuppressant therapy, as well as patients who lost response or were intolerant to anti-TNF agents.§ Two treatment arms were evaluated. Co-primary endpoints: percentage of patients achieving clinical remission at Weeks 8 and 52.1,3
TNF=tumor necrosis factor
Also Check: Yea Sacc For Horses With Ulcers