Treatment Options For Ulcerative Colitis
Your treatment options for UC will depend on the severity of the condition. In most cases, medications can help reduce symptoms and inflammation. The most common medications include:
- 5-aminosalicylic acid
- Corticosteroids typically reserved for moderate or severe UC
- Immunomodulator drugs
If your condition is life-threatening, your doctor may suggest surgery to remove the entire colon and rectum. An ileal pouch-anal anastomosis is a procedure that adds a small bag to the end of your small intestine that connects to your anus making expelling waste relatively normal.
When other medications dont seem to be working, and surgery isnt yet an option, biologics can provide a relatively safer and more effective solution. Biologic agents are drugs administered intravenously and can provide relief by reducing inflammation and other symptoms.
Assessment Of Dose Escalation
Dose escalation was defined differently for each biologic therapy, although the principle was the same. First, an induction period was calculated for each therapy, based on the labeled induction length, with an extra 1 week added for adalimumab and golimumab, and an extra 2 weeks added for infliximab and vedolizumab, to account for slight delays or variation in administration. Induction doses for adalimumab , golimumab , and vedolizumab were ignored for the purpose of calculating dose escalation . If the average dose during maintenance was at least 20% higher than the product label, this was considered dose escalation. For infliximab, since dosing is weight-based and weight was not available from the claims data, the index dose was used to derive an average daily baseline maintenance dose. This was calculated by dividing the total quantity administered at index by 56 . If this estimated average dose during maintenance was at least 20% higher than the index dose, this was considered dose escalation. For all medications, dose escalation was evaluated as the only persistent portion of the maintenance period because the gap might signal a treatment interruption.
Biological Therapy For Ulcerative Colitis: An Update
Correspondence to: Soo-Cheon Chae, PhD, Professor, Department of Pathology, Wonkwang University School of Medicine, Digestive Disease Research Institute, 460 Iksandae-ro, Iksan, Cheonbuk 570-749, South Korea.
Telephone: +82-63-8506793 Fax: +82-63-8522110
You May Like: Surgical Management Of Ulcerative Colitis Ppt
Why Have I Been Prescribed Biologics For Inflammatory Bowel Disease
Biologic medicines are relatively new, but have been shown to be effective in treating and maintaining remission in people with moderate to severe Crohns disease and ulcerative colitis. They may be an option when other medicines, such as immunosuppressants and steroids havent been effective.
However, biologics dont work for everyone and can also stop working after youve been taking them for a while.
Reduces Inflammation From The Inside
ZEPOSIA treats ulcerative colitis by reducing inflammation below the surface and improving the appearance of the colon at 1 year. ¶
¶30% of people taking ZEPOSIA achieved this result compared to 14% taking the placebo.
Early symptom relief and lasting remission are possible for my UC patients with this once-daily pill.
Douglas C. Wolf, MD
- High blood pressure
- Headache
These are not all the possible side effects of ZEPOSIA. Please see the full Prescribing Information and Medication Guide for information on all of the side effects reported by those taking ZEPOSIA.
Read Also: Best Medicine For Ulcerative Colitis
Recommended Reading: What To Do For Mouth Ulcers
Interactions With Other Medications
Interactions between biologics and other medications can be significant, and each type of biologic drug can present different risks.
A doctor should be aware of all the over-the-counter or prescription medications, and all the supplements, herbal medications, and vitamins that a person takes before they prescribe a biologic.
Anyone using a biologic for UC should speak with a doctor before receiving a vaccine. For people who use certain biologic medications, including golimumab, infliximab, and adalimumab, having a live vaccine can be dangerous, and doctors recommend avoiding it.
, this type of treatment costs $10,00030,000 per year, on average, and the more expensive types can cost more than $500,000 annually.
A doctor may instead recommend a type of drug called a biosimilar. There is very little clinical difference between these drugs and biologics. Biosimilars are less expensive but just as safe and effective.
The Food and Drug Administration approved the use of biosimilars in an effort to reduce costs. However, the FDA do not regulate whether insurance companies cover the costs of these drugs.
The following table provides an overview of the biologics available to treat UC. The abbreviation IV stands for intravenous.
| Drug |
Promising Jak1/jak3 Inhibitor And Integrin Blocking Antibody
The JAK-STAT pathway is associated with inflammation, autoimmune diseases, hematopoietic disorders, and transplant rejection. Tofacitinib is a selective oral inhibitor of JAK 1 and 3, which is known to inhibit the differentiation of pathogenic Th1 and Th17 cells and innate immune cell signaling. The effects of tofacitinib in active UC patients showed clinical response rates at week 8 of 32%, 48%, 61%, and 78% for the 0.5 mg, 3 mg, 10 mg, and 15 mg twice daily groups, respectively. The tofacitinib 3 mg, 10 mg, and 15 mg twice daily groups exhibited marked differences in clinical and endoscopic remission rates compared to the placebo group . The levels of low-density and high- density lipoprotein cholesterol increased in a dose-dependent manner, and an absolute neutrophil count of < 1500 was observed in 2% of patients in the tofacitinib group. Thus, tofacitinib is considered to be an effective and safe drug for moderate-to-severe UC patients.
Read Also: How To Test For Ulcerative Colitis
Can I Prevent Ulcerative Colitis
There is currently no known way to prevent or cure for ulcerative colitis but the proper strategy for managing your disease can help you lead a happier, healthier, fulfilling life.
The exact cause of ulcerative colitis is unknown. However, it is believed to be due to a combination of factors, including a persons genes and triggers in the environment. This interaction of genetic and environmental factors activates an abnormal response of the bodys immune system.
Normally, the immune system protects the body from infection. In people with ulcerative colitis, however, the immune system can mistake microbes , food, and other material in the intestines, as invading substances.
When this happens, the body launches an attack, sending white blood cells into the lining of the intestines where they cause inflammation and ulcerations.
Approval And Usage Status Of Biologics
The biologics which can be used in ulcerative colitis are different according to the countries. Golimumab was approved in the European Union, Canada, Switzerland, Russia, the United States, South Korea, and other countries.
In the United Kingdom, only infliximab is recommanded in the acute severe ulcerative colitis according to NICE guideline. Other European countries such as Greece, France, Italy, Spain, Sweden, etc., infliximab, adalimumab, and golimumab are available for treatment of moderately to severely active ulcerative colitis in adult patients who have had an inadequate response to conventional therapy including corticosteroids and 6-MP or azathioprine, or who are intolerant to or have medical contraindications for such therapies. Recently, vedolizumab was approved for moderate to severe ulcerative colitis by the EMA.
In the United States, infliximab, adalimumab, and golimumab are available in patients with moderately to severely active disease who have had an inadequate response to conventional therapy. The FDA approved vedolizumab recently.
In Japan, infliximab and adalimumab can be used in patients with moderate to severe ulcerative colitis inadequate response to conventional therapy.
The biologics which can be used for ulcerative colitis in South Korea are infliximab and adalimumab. Golimumab is expected to be available soon in South Korea.
Recommended Reading: How To Get Rid Of Tongue Ulcers
Side Effects And Risks
Because Inflectra is a biosimilar of infliximab , Inflectra and Remicade have the same side effects. Examples of common and serious side effects for each drug are listed below.
More common side effects
This list contains more common side effects that can occur with both Remicade and Inflectra :
for these side effects. A boxed warning is the most serious warning from the Food and Drug Administration . For more information, see at the beginning of this article.
Inflectra For Crohns Disease
Inflectra is FDA-approved to treat moderate to severe Crohns disease in adults and children ages 6 years and older. Its approved for use when other medications havent worked well enough.
Crohns disease is a type of IBD. It can affect any part of your digestive tract, from your mouth to your anus. Common symptoms include:
- abdominal pain
- weight loss
- anemia
Severe Crohns disease can sometimes cause fistulas.
Inflectra is prescribed to induce remission of Crohns disease. Its also prescribed to maintain remission .
Inflectra is also prescribed to help heal fistulas in adults. These include fistulas that have formed between the rectum and vagina or between the intestines and the skin. And Inflectra can help prevent healed fistulas from opening again.
To learn more about Crohns disease and its treatment, you can visit our IBD hub.
Effectiveness for Crohns disease
Inflectra has been found effective for treating Crohns disease. The American College of Gastroenterology guideline recommends infliximab-dyyb as a treatment option for Crohns disease.
For information on how the drug performed in clinical trials, see Inflectras prescribing information.
Also Check: What Are Ulcers In Horses
What Differences Might There Be
Biosimilars are thoroughly tested. They meet strict standards to show they are as safe, effective and have no clinically meaningful differences from the originator. Where NICE has recommended the use of a biological medicine, they state that the same guidance applies to the biosimilar.However, there are likely to be some small differences including:
Some people may be sensitive to latex, which is used as a needle cover in some of some types of biosimilars, or citrate which is sometimes included as one of the ingredients.Some types of adalimumab are available without citrate or latex, and you can ask to try one of these.
Can Inflectra Cause Hair Loss

No, hair loss isnt likely. Hair loss wasnt reported in clinical trials of Inflectra.
However, some cases of new or worsening psoriasis* have been reported in people receiving Inflectra treatment. Psoriasis can cause an itchy rash, or raised, scaly patches of skin. If psoriasis affects your scalp, this could cause clumps of hair loss. If psoriasis affects other parts of your body, it could cause hair loss there as well.
If you have hair loss during Inflectra treatment, talk with your doctor. They can determine the possible cause.
* Inflectra is approved to treat in adults. For more information, see the section below.
Don’t Miss: What Not To Eat With Bleeding Ulcers
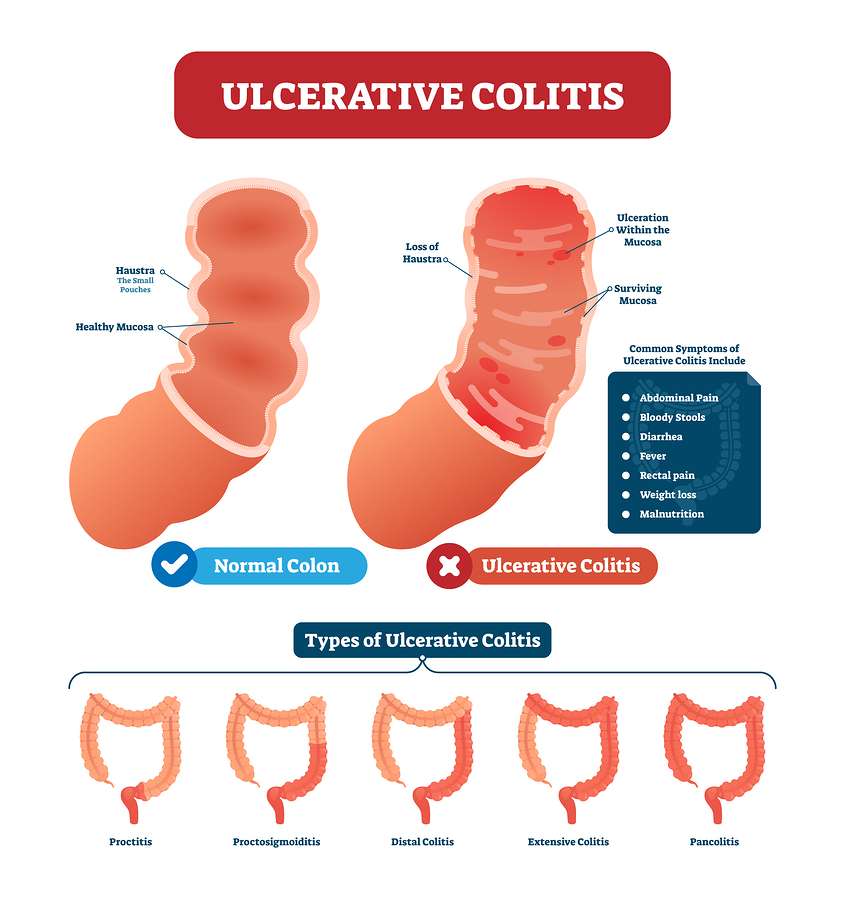
Biological Treatment For Ulcerative Colitis
Stomach aches can be a nuisance did you catch a bug, are you cramping, or is it the sign of something else? For about 750,000 Americans, the cause is ulcerative colitis . The condition is debilitating and can lead to life-threatening complications if left untreated. Fortunately, biological treatment is providing relief for those suffering from the disease and looking for alternatives to medications.
Inflectra For Ulcerative Colitis
Inflectra is FDA-approved to treat moderate to severe ulcerative colitis in adults and children ages 6 years and older. Its approved for use when other medications havent worked well enough.
UC is a type of inflammatory bowel disease that affects your colon and rectum. Common symptoms include abdominal pain or cramps and diarrhea that may contain blood or mucus.
Inflectra is prescribed to induce remission of UC. Its also prescribed to maintain remission .
To learn more about UC and its treatment, you can visit our IBD hub.
Effectiveness for UC
Inflectra has been found effective for treating UC. The American College of Gastroenterology guideline recommends infliximab as a treatment option for UC. Infliximab-dyyb, the active drug of Inflectra, is a version of infliximab. So Inflectra is just as safe and effective as infliximab for UC.
For details on how the drug performed in clinical trials, see Inflectras prescribing information.
Read Also: Natural Enemas For Ulcerative Colitis
Tofacitinib: New Drug Approved For The Treatment Of Patients With Ulcerative Colitis
Ulcerative colitis is a chronic inflammatory disease that affects the colonic mucosa. There is no curative treatment. Instead, patients must take medication to control the inflammation over the course of their lives. There are some patients whose condition cannot be sufficiently controlled by the drugs currently available. For them, the new treatment with tofacitinib could be an alternative.
In 2017, the European Medicines Agency approved tofacitinib for the treatment of ulcerative colitis in adult patients who presented an intolerance, insufficient response or loss of response to conventional treatment or biological medical products , which are also commonly administered to treat ulcerative colitis.
Tofacitinibs mechanism of action is different to all other currently approved molecules: it inhibits a route of inflammation known as JAK . Inhibiting this route of action represents a new approach in the treatment of ulcerative colitis.
This treatment has many advantages in terms of its administration: it is administered orally, and this encourages patients to accept and follow the treatment. Furthermore, it has a fast onset of action, the effect of the drug only lasts for a few hours in the body, there is no risk of producing antibodies against it and it is used in monotherapy, in other words, there is no need to combine it with other drugs.
- Keep reading about:
Routine Tests To Get Started
You can start treatment after taking 2 routine tests that can help make sure ZEPOSIA is right for you. If you have a history of certain eye conditions , you may also need an eye exam.
If youve had some of these tests within the last 6 months, let your healthcare provider knowthey may not need to be repeated.
Your UC Nurse Navigator or healthcare provider will help schedule these routine tests. For eligible patients, they may all be completed with an in-home visit:*
-
An electrocardiogram a common test that uses small sensors to monitor your heart and makes sure its working normally before you start treatment.
-
Blood workincluding complete blood count and liver function tests.
The ZEPOSIA Prescribing Information does not require ongoing lab monitoring unless indicated by your healthcare provider.
Once I was prescribed ZEPOSIA, a clinician from ZEPOSIA 360 Support was sent to my house to do all the necessary tests.*
Trey
You May Like: Peptic Ulcer Treatment At Home
Prednisone Prednisolone And Methylprednisolone
Prednisone is taken by mouth and is available as:
- an immediate-release tablet
- a delayed-release tablet
- a liquid solution
Its available as a generic drug and as the brand-name drugs Prednisone Intensol and Rayos .
The forms of prednisolone that are FDA approved for UC are:
- immediate-release tablet
- liquid solution
- syrup
You can take any of these forms by mouth. Prednisolone is available as a generic drug and as the brand-name drugs Millipred and Prelone .
Methylprednisolone comes in two forms:
- oral tablet
- injectable medication
Its available as a generic drug and as the brand-name drugs Medrol and Depo-Medrol .
Side effects, complications, and interactions
When given in high doses, the side effects of these drugs are practically indistinguishable. The more common side effects can include:
- increased blood sugar levels
Immunomodulators are drugs that decrease the bodys response to its own immune system. The result is lowered inflammation throughout your body.
Immunomodulators may reduce the number of UC flare-ups you have and help you stay symptom-free longer.
Theyre generally prescribed to people whose symptoms havent been managed with 5-ASA drugs and corticosteroids. However, these drugs may take several months to start working.
The Food and Drug Administration hasnt approved immunomodulators for the treatment of UC.
However, theyre well supported in medical literature as useful options, and your doctor may still prescribe them. This is known as off-label drug use.
What Is A Biosimilar
Sometimes you will be prescribed a biosimilar. Biosimilars are very close copies of the original brand of biologic medicine . They are thoroughly tested to make sure they are as safe and effective, and work in the same way, as the originator.
Other companies can start making copies of the original medicine when it comes off patent. So far this has happened with infliximab and adalimumab.
Find out more about biosimilars.
Don’t Miss: Cheap Ulcer Treatment For Horses
Biologics May Lower The Need For Surgery
Biologics can help protect some patients with moderate to severe ulcerative colitis from requiring surgery or hospitalization. The percentage of people with ulcerative colitis receiving biologic drugs has increased substantially since 1998, when infliximab became the first FDA-approved biologic. Now, about 16% of the patient population is estimated to use biologics.
According to a 2020 study in Inflammatory Bowel Disease of more than 500 patients with UC, the introduction and utilization of biologics may be responsible for a marked decline in the number of patients who need to undergo surgery.
In the pre-biologics era, about 20% of patients with ulcerative colitis needed a colectomy during their first hospitalization, and 30% required a colectomy within a year of their first hospitalization.Since the introduction of biologics, those rates have declined to 5.3 and 11.9%, respectively, providing potential evidence that biologics have spared many patients from losing their colons.
Steroids For Ulcerative Colitis Treatment

Steroid use is often one part of the treatment plan for ulcerative colitis.
Most healthcare providers will prescribe steroids to treat a flare-up of symptoms, then work to lower the dose as early as possible. This helps avoid negative side effects such as dependence, weight gain, and effects on blood sugar regulation.
Read Also: Does Tylenol Cause Stomach Ulcers
Don’t Miss: How Can Ulcers Be Treated