Ozanimod Improves Ulcerative Colitis Symptoms
A phase 3 study involving 645 patients with moderate to severe ulcerative colitis showed treatment with ozanimod improved several critical symptoms, including rectal bleeding and stool frequency.
The True North study evaluated the efficacy of the ozanimod versus placebo on those two symptoms, as well as fecal calprotectin and C-reactive protein during the induction phase, with the researchers assessing how quickly the patients saw improvement.
According to results presented at the virtual Digestive Disease Week meeting, the baseline rectal bleeding scores were 1.7 and 1.6 in the drug and placebo arms, respectively. The mean baseline stool frequency score was 2.4 in both groups.
The researchers noted improvement in rectal bleeding was seen starting at week 2 and SFS at week 5 . At week 10, 52% of patients in the ozanimod arm reported no rectal bleeding compared to 30.1% in the placebo arm . More improvement was also seen in stool frequency .
The mean change in FCP concentration at week 10 was -470.2 µg/g with ozanimod and 21.1 µg/g with placebo . The mean change in CRP from baseline to weeks 5 and 10 were also greater with ozanimod than placebo .
Read Also: Nursing Care Plan For Pressure Ulcer Prevention
Fda Approves Ozanimod For Adults With Ulcerative Colitis
The treatment is also being studied for safety and efficacy in patients with Crohns disease.
The US Food and Drug Administration has approved ozanimod 0.92 mg, an oral agent that selectively targets sphingosine-1-phosphate receptor subtypes 1 and 5, for adult patients with moderately to severely active ulcerative colitis.
The approval, awarded to Bristol Myers Squibb, was based on the data from a placebo-controlled phase 3 trial dubbed True North. In the study, researchers evaluated ozanimod as a single, daily oral therapy for both adults and pediatric patients at least 12 years old with moderately to severely active ulcerative colitis.
Despite the availability of approved therapies, there is still unmet need and an opportunity to deliver additional treatment options to help patients better manage their disease, said Adam Lenkowsky, general manager and head, U.S., Cardiovascular, Immunology and Oncology, Bristol Myers Squibb, in a statement. Were thrilled that our pursuit of transformative science in immunology may benefit patients in their ulcerative colitis treatment by introducing a new option that has a different mechanism of action than available therapies. Zeposia combines disease control through lasting remission and demonstrated safety in a once-daily pill.
Ozanimod Yields Clinical Response Remission In Ulcerative Colitis Patients
Serious infections occurred in less than 2% of patients treated with the medication during the duration of the 52-week trial.
Results from both an induction and maintenance therapy trial show ozanimod can be an effective treatment for patients with inflammatory bowel disease .
A team, led by William J. Sandborn, MD, University of California San Diego, compared ozanimod with placebo in achieving both clinical remission and clinical response in patients with moderate to severely active ulcerative colitis.
Ozanimod is a selective sphingosine-1-phosphate receptor modulator currently being studied for the treatment of patients with IBD.
Don’t Miss: How To Treat Diabetic Ulcers On Toes
Bristol Myers Squibb Presents Interim Results From Long
The percentage of patients achieving clinical remission, clinical response, endoscopic improvement and corticosteroid-free remission was maintained through Week 142
Zeposia is the first and only oral sphingosine 1-phosphate receptor modulator approved to treat patients with ulcerative colitis
PRINCETON, N.J.â-Bristol Myers Squibb today announced interim results from the True North open-label extension study evaluating the long-term efficacy and safety profile of Zeposia in patients with moderately to severely active ulcerative colitis . Findings show that the percentage of patients achieving clinical remission, clinical response, endoscopic improvement and corticosteroid-free remission was maintained through Week 142. No new safety signals emerged in the study. These data will be presented at the 17th Congress of the European Crohnâs and Colitis Organisation , taking place February 16-19, 2022.
Additional Bristol Myers Squibb-sponsored abstracts presented at the ECCO 2022 Congress can be accessed online here.
Visit this page on BMS.com for more information on Bristol Myers Squibbs scientific approach and resources on gastrointestinal immune-mediated diseases.
About True North
Bristol Myers Squibb thanks the patients and investigators involved in the True North clinical trial.
About Ulcerative Colitis
ZEPOSIA is indicated for the treatment of:
IMPORTANT SAFETY INFORMATION
About Bristol Myers Squibb
Dop69 Ozanimod For Induction Treatment Of Moderate
Eaton, K. Duperrouzel, C. Bhandari, P. Craigie, S. Bonner, A. Cameron, C. Tencer, T. Kumar, J.
CRG-EVERSANA, Value & Evidence Division- Marketing and Market Access, Ontario, Canada CRG-EVERSANA, Value & Evidence Division- Marketing and Market Access, Sydney- Nova Scotia, Canada Bristol Myers Squibb, Global HEOR, Princeton, United States
You May Like: What Is The First Sign Of Stomach Ulcer
Also Check: How Do Doctors Test For Ulcerative Colitis
A Phase 3 Multicenter Randomized Double
Clinical Trial Details
YELLOWSTONE Induction Study : This study is designed to determine the safety and effectiveness of the oral investigational study drug, ozanimod, versus a placebo in achieving symptom remission in patients with active Crohnâs disease symptoms. An induction study is the first in a series of studies. Participation in this clinical study is expected to last 12 weeks .
Depending on response and the study doctorâs recommendation, participants may have the opportunity to continue participation in the YELLOWSTONE Maintenance RPC01-3203 or YELLOWSTONE Open-Label Extension Study . An open label extension study means you may be able to continue taking the investigational study drug, if you qualify and choose to participate
Ozanimod is thought to act on the immune system by encouraging certain types of white blood cells called lymphocytes, which include T cells, to stay in the lymph nodes and other places in the body, thereby keeping them away from sites of inflammation.
Lymphocytes, which act as the bodyâs mechanism to fight off invaders, are responsible for initiating the immune response. However, in Crohnâs disease, lymphocytes misread the inflammation caused by the disease as an area where their help is needed.
You may be able to take part in this ozanimod study if you:
Eligible prior medications include corticosteroids, immunomodulators or biologic therapy .
The presence of any of the following will exclude you from participation in the study:
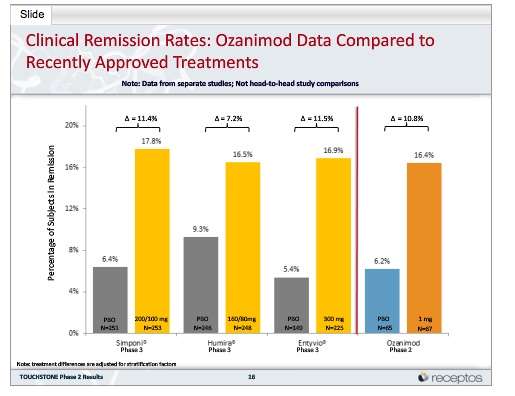
Clinically Meaningful Improvements With Ozanimod In Uc Trial
The phase 3 True North trial evaluating the efficacy of ozanimod as an induction and maintenance therapy for adults with moderate to severe ulcerative colitis met both primary and key secondary end points, according to Bristol Myers Squibb.
Ozanimod is a sphingosine 1-phosphate receptor modulator marketed under the brand name Zeposia®. The product is currently approved for the treatment of relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
The multicenter, double-blind, placebo-controlled True North study included patients with moderate to severe ulcerative colitis who had an inadequate response to prior treatment. In the induction phase, patients were randomized to receive either ozanimod 1mg orally once daily or placebo for 10 weeks. Patients who achieved a clinical response in the induction phase or who were part of an open-label arm were eligible to proceed into the maintenance phase, in which they were re-randomized to receive ozanimod or placebo through week 52.
Results showed that ozanimod met both primary end points achieving statistically significant clinical remission at week 10 in the induction phase and at week 52 in the maintenance phase .
Recommended Reading: What Can I Do For Ulcer Pain
Efficacy And Safety Of Combination Induction Therapy With Guselkumab And Golimumab In Participants With Moderately To Severely Active Ulcerative Colitis: Results Through Week 12 Of A Phase 2a Randomized Double
Guselkumab is an antagonist of the p19 subunit of IL-23 that is approved to treat plaque psoriasis. Golimumab is an antagonist of TNF and is approved for the treatment of ulcerative colitis.1 The phase 2a VEGA study evaluated the safety and efficacy of induction therapy with guselkumab plus golimumab vs monotherapy with guselkumab or golimumab in adults with moderately to severely active ulcerative colitis.2The trial enrolled patients with a Mayo score of 6 to 12 and an endoscopy subscore of 2 or lower according to central review. The patients had received prior therapy that was either intolerable or unsuccessful. The trial excluded patients previously treated with a TNF antagonist.
Patients were randomly assigned into the 3 arms. In the golimumab monotherapy arm, this agent was administered at 200 mg subcutaneously at week 0, followed by 100 mg administered at weeks 2, 6, and 10. In the guselkumab monotherapy arm, treatment was administered at 200 mg intravenously at weeks 0, 4, and 8. For combination therapy, the 2 antibodies were administered in combination at the same doses and schedules. The induction phase for all 3 arms continued for 12 weeks. The primary endpoint was clinical response, defined as a decrease from baseline in the Mayo score of at least 30% and 3 points, with either a decrease in the rectal bleeding subscore of 1 or more or a rectal bleeding subscore of 0 or 1.
References
Ozanimod Treatment Shows Maintained Response In Ulcerative Colitis
- Presented By
- Prof. Silvio Danese, Vita-Salute San Raffaele University, Italy
- Conference
- Trial
- Phase 3, True North
Ozanimod demonstrated long-term durability of efficacy in participants with active ulcerative colitis . Moreover, results from the open-label, extension study of the phase 3 True North trial did not display new safety issues associated with the long-term use of ozanimod.Ozanimod is an S1P receptor modulator, approved for the treatment of patients with moderately to severely active UC based on the 52-week results of the True North trial . An open-label extension study was initiated to assess the long-term efficacy of ozanimod . In total, 823 participants entered the open-label extension study of the True North trial, of whom 64%, 34%, and 14% completed the week 46, week 94, and week 142 time points, respectively. Lack of efficacy and withdrawal by subject were the main reasons for treatment discontinuations. Prof. S…
Please login to read the full text of the article.
If you have no account yet, please register now.
Don’t Miss: What Doctor Treats Ulcerative Colitis
Pk Of Ozanimod With Gemfibrozil Itraconazole Or Rifampin
This phase 1, randomized, open-label study focused on assessing the single-dose pharmacokinetics of ozanimod and its metabolites as well as to assess the effects of gemfibrozil, itraconazole, and rifampin on the single-dose PK of ozanimod. A total of 40 patients were randomized to receive either a single oral dose of ozanimod, oral doses of gemfibrozil + a single dose of ozanimod, oral doses of itraconazole + a single dose of ozanimod, or oral doses of rifampin + a single dose of ozanimod. In the single dose of ozanimod alone group, there were dose-proportional increases in Cmax and AUC for both the parent drug, ozanimod as well as its metabolites CC112273 and CC1084037. Itraconazole, a strong inhibitor of CYP3A and P-glycoprotein increased ozanimod AUC by 13%, while rifampin, a strong inducer of CYP3A and P-gp, reduced the AUC of ozanimod by 24%. This implies that there is a CYP3A4 and P-gp involvement in the metabolism of ozanimod. Gemfibrozil, a strong inhibitor of the CYP450 system, increased the AUC for the metabolites of ozanimod, CC112273 and CC1084037 by 47% and 69%, respectively. The metabolites of ozanimod were found to have similar single-dose PK properties, with CYP2C8 being the main enzyme in the metabolism of these metabolites, and CYP3A4 and P-gp being enzymes for the metabolism of ozanimod .
L.R. Fitzpatrick, T. Woldemariam, in, 2017
Ozanimod Is An Efficacious Oral Therapy After 5
A post hoc analysis of data from the phase 3 True North trial assessed the efficacy of 10 weeks of ozanimod induction therapy, with or without concomitant corticosteroid treatment.1,2 The patients had received prior treatment with 5-aminosalicylic acid, but not with immunomodulators or biologic therapies. Bruce Sands, MD, presented the results.1 Among 464 enrolled patients, 101 received placebo and 205 received ozanimod in cohort 1, while 158 received open-label ozanimod in cohort 2.
Among all patients in the analysis, clinical remission at week 10 was reported in 23.4% of cohort 1, 30.4% of cohort 2, and 8.9% of the placebo arm . A clinical response occurred in 53.7% of cohort 1, 62.7% of cohort 2, and 30.7% of the placebo arm . Endoscopic improvement was reported in 35.6% of cohort 1, 38% of cohort 2, and 14.9% of the placebo arm . Mucosal healing occurred in 18% of cohort 1, 14.6% of cohort 2, and 5.0% of the placebo arm .
At week 10, in cohort 1, the rate of clinical remission was 19.0% with ozanimod vs 5.0% with placebo among the patients receiving concomitant corticosteroids. Among patients who were not receiving corticosteroids, clinical remission was reported in 24.5% of the ozanimod arm vs 9.9% of the placebo arm . The rate of clinical response was 59.5% with ozanimod vs 30.0% with placebo in patients who concomitantly used corticosteroids , and 52.1% vs 30.9% in those who did not.
References
Also Check: Can Stomach Ulcers Cause Cancer
Patient Disposition And Baseline Characteristics
Of 197 patients in TOUCHSTONE, 170 entered the OLE period and received daily ozanimod HCl 1 mg . Of these, 81 patients entered the OLE period at the end of the induction period, seven entered during the maintenance period, and 82 entered at the end of the maintenance period . At the time of this analysis , 99 patients had discontinued the OLE study, with 28% of the patients discontinuing in the first year, and an annual discontinuation rate of 15 18% for existing patients in years 24 . Reasons for discontinuation are shown in . At study closure, of the 71 UC patients eligible to roll over into the phase 3 OLE study, 54 did so as a joint decision of patients and the treating investigators .
Patient disposition.
*In 2019, the sponsor made the decision to close the phase 2 TOUCHSTONE study after all active patients had completed at least 4 years of follow-up.
Of the 170 patients in the OLE, the mean age of patients at baseline for the double-blind study was 40.4 years, 57.6% of patients were male and 92.4% of patients were white . The mean duration of disease from diagnosis to the baseline of the double-blind study was 5.9 years, and most patients had not received prior anti-tumour necrosis factor therapy.
The mean exposure to ozanimod over the course of the study was 2.8 person-years .
Ozanimod Maintenance In Patients With Ulcerative Colitis Associated With Corticosteroid
|
The following article is a part of conference coverage from the Digestive Disease Week 2021 Annual Meeting , held virtually from May 21 to 23, 2021. The team at Gastroenterology Advisor will be reporting on the latest news and research conducted by leading experts in gastroenterology. Check back for more from DDW 2021. |
For patients with ulcerative colitis, an increased likelihood of achieving corticosteroid-free remission is associated with maintenance treatment with ozanimod vs placebo. This was reported in data presented at the Digestive Disease Week 2021 annual meeting.
Investigators performed further analysis of patients in the maintenance phase of the True North phase 3 trial. Patients with a clinical response to ozanimod at week 10 of the induction phase were re-randomized to either maintenance with 1 mg of ozanimod or placebo. Corticosteroid doses were kept stable during induction then tapered as tolerated during maintenance.
Roughly 33% of patients were on concomitant systemic corticosteroids at baseline. By week 10, 18.4% and 6.0% of the ozanimod and placebo groups achieved clinical remission while on stable corticosteroids, respectively . In the re-randomized maintenance population, 37.0% of the 230 patients in the ozanimod group and 18.5% of the 227 patients in the placebo group achieved clinical remission at week 52 . There was also a higher proportion of patients in corticosteroid-free remission in the ozanimod group vs placebo at week 52 .
Reference
Also Check: What Foods To Avoid When You Have An Ulcer
Q: What Are The Limitations Of Current Therapies For Ulcerative Colitis
A: Conventional therapies such as aminosalicylates are modestly effective in patients with moderate, but not severe, disease. Glucocorticoids have been associated with adverse events and long-term adverse health consequences and are not recommended as maintenance therapy. Newer agents, including biologic drugs and Janus kinase inhibitors, are not effective in all patients or can lose efficacy with long-term use, and they have been associated with infections, infusion reactions, and cancers. Thus, the need remains for new oral treatments for ulcerative colitis that are safe and glucocorticoid-sparing and that have durable efficacy.
A Study Investigating Oral Ozanimod In Pediatric Participants With Moderate To Severe Active Ulcerative Colitis
| The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government.Know the risks and potential benefits of clinical studies and talk to your health care provider before participating. Read our disclaimer for details. |
| First Posted : October 13, 2021Last Update Posted : June 21, 2022 |
| Study Type : | |
| Quadruple | |
| Primary Purpose: | Treatment |
| Official Title: | A Phase 2/3, Multicenter, Randomized, Double-Blind Study to Evaluate the Efficacy, Safety, Pharmacokinetics and Pharmacodynamics of Oral Ozanimod in Pediatric Subjects With Moderately to Severely Active Ulcerative Colitis With an Inadequate Response to Conventional Therapy |
| Actual Study Start Date : |
| Specified dose on specified days | |
| Experimental: Ozanimod Low Dose | Specified dose on specified days |
Exclusion Criteria:
Read Also: Can I Take Tylenol For Stomach Ulcer Pain