Alternatives For Moderate To Severe Crohns Disease
According to treatment guidelines, other drugs that may be used to treat Crohns disease include:
- oral corticosteroids, such as prednisone
- azathioprine
Humira is also FDA-approved to treat:
Remicade and Humira are considered treatment options for people whose disease is moderate to severe. For Crohns disease, ulcerative colitis, and plaque psoriasis, the drugs are usually prescribed for people who tried other medications that didnt ease their symptoms. For more information on how Remicade is used, see the Remicade uses section below.
What Should I Ask My Doctor
The sections above describe the typical dosages provided by the drug manufacturer. If your doctor recommends Remicade for you, theyll prescribe the dosage thats right for you.
Remember, you wont give yourself Remicade doses. Youll receive the infusions at your doctors office or an infusion clinic. Talk with your doctor if you have questions or concerns about your current dosage.
Here are some examples of questions you may want to ask your doctor:
- Would a different dosage raise or lower my risk for side effects from Remicade?
- Will I have to receive my Remicade doses at a special infusion center?
- What should I expect during my Remicade infusion?
You can subscribe to the Healthline newsletters for psoriasis or rheumatoid arthritis if you use Remicade for either of these conditions.
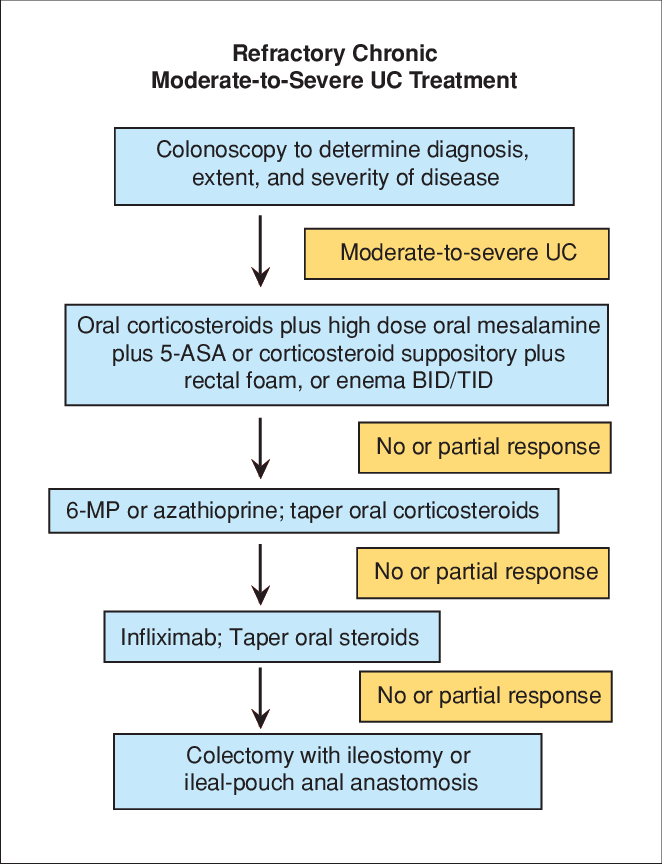
Remicade Treats Ulcerative Colitis
Better Results Than With Fake Drug, Researchers Report
Dec. 7, 2005 — The rheumatoid arthritis drug Remicade may help treat moderate-to-severe ulcerative colitis, a new study shows.
WebMD first reported the news in May, when findings were presented at a medical conference. Now, more details appear in The New England Journal of Medicine.
The researchers included Paul Rutgeerts, MD. He works in Leuven, Belgium, at the University Hospital Gasthuisberg.
Ulcerative colitis is an inflammatory bowel disease that primarily affects the colon and rectum with inflammation and ulcers leading to bleeding and abdominal pain. The disease generally follows a course of flare-ups that can be difficult to manage. In some circumstances, surgery may be necessary to remove the affected area.
Also Check: What Can You Eat If You Have Ulcerative Colitis
Remicade Generic Or Biosimilar
Remicade is available only as a brand-name medication. It contains the active drug ingredient infliximab.
The Food and Drug Administration has approved four biosimilar versions of Remicade: Avsola, Inflectra, Ixifi, and Renflexis.
A biosimilar is a medication thats similar to a brand-name drug. A generic medication, on the other hand, is an exact copy of the active ingredient in a brand-name drug. Biosimilars are based on biologic medications, which are created from parts of living organisms. Generics are based on regular drugs made from chemicals. Biosimilars and generics also tend to cost less than brand-name medications.
Route Of Administration Of Methotrexate And Clinical Effectiveness

There is ongoing debate about possible differences in the bioavailability of methotrexate given parenterally or orally. Pharmacokinetic studies employing methotrexate suggest that in the dose range of 1525 mg an oral application results in a significantly lower bioavailability of the drug, at least in a subgroup of patients.26,27,36,37 Interestingly, in a prospective, double-blind, controlled trial comparing an oral and an s.c. regimen of methotrexate in patients with rheumatoid arthritis, a significantly higher response rate in patients treated s.c. with methotrexate was observed compared to the orally treated group.38 Similarly, in a retrospective study of children with juvenile idiopathic arthritis who were treated with orally administered methotrexate in doses up to 20 mg weekly, an improved efficacy was reported when the oral administration of methotrexate was changed to a parenteral application.39
Overall, the CD data, demonstrating no significant effect in patients treated with oral doses of methotrexate between 12.522.5 mg/week8,9,16 and very good efficacy of the parenteral administration of doses of 1525 mg/week for both induction or maintenance6,7,42 also indicate that in addition to the dose the route of administration may represent a crucial factor at least in patients with CD.
Don’t Miss: I Think I Have An Ulcer What Should I Do
Remicade Drug Class And Form
Remicade contains the drug infliximab, which is a biologic . Remicade belongs to a drug class called tumor necrosis factor-alpha blockers. A class of drugs is a group of medications that work in a similar way.
Remicade comes as a vial of powder thats mixed with a liquid solution. The drug is available in one strength: 100 mg.
A healthcare provider will give you Remicade as an infusion. This is an injection into your vein thats given over a period of time. Remicade infusions are typically about 2 hours long. Youll usually receive an infusion every few weeks, but the timing depends on the condition thats being treated.
Safety And Adverse Events
There were 59 infectious events experienced by 31 patients on HD IFX therapy. Respiratory infections were the most common consisting mainly of nonspecific viral syndromes and sinus infections . However, two patients required hospitalization for pneumonia and 24 infectious events occurred on combination therapy with an immunomodulator. No mycobacterial infections, demyelinating events, or instances of new-onset heart failure were documented.
The incidence rates of serious infections are stratified by average weekly IFX dose in . Five serious infections occurred among five patients receiving 10mg/kg every 4 weeks, and five serious infections occurred among two patients receiving 20mg/kg every 4 weeks. One of the patients receiving 20mg/kg every 4 weeks for fistulizing Crohns disease also had axillary and perianal hidradenitis suppurativa, had declined recommended surgical therapy and was frequently noncompliant with prescribed medical therapy. During an 18-month period of compliance with HD IFX she experienced four serious infections: two perianal abscesses requiring drainage, one central line infection, and one pneumonia.
You May Like: How Do You Diagnose A Stomach Ulcer
Impact 2 Study Design56
IMPACT 2 : a randomized, double-blind, placebo-controlled, multicenter, phase 3, parallel-group study of REMICADE® in 200 adult patients with active PsA for at least 6 months who had an inadequate response to disease-modifying antirheumatic drugs or nonsteroidal anti-inflammatory drugs . Patients had active articular disease , psoriatic target skin lesion , and either C-reactive protein â¥1.5 mg/dL or morning stiffness lasting â¥45 minutes. Stable methotrexate doses of â¤25 mg/week at study entry and stable oral corticosteroid doses equivalent to â¤10 mg/day of prednisone were permitted. During the 24-week, double-blind phase, patients received either REMICADE® 5 mg/kg IV or placebo at Weeks 0, 2, 6, 14, and 22. At Week 16, placebo patients with < 10% improvement in swollen and tender joint counts were switched to active treatment and received REMICADE® 5 mg/kg IV at Weeks 16, 18, 22, 30, 38, and 46. At Week 24, all patients receiving placebo crossed over to active treatment and received REMICADE® 5 mg/kg IV at Weeks 24, 26, 30, 38, and 46. Primary endpoints included the proportion of patients with ACR20 response at Week 14 and the change from baseline in total modified van der Heijde-Sharp score at Week 24. Improvement in Psoriasis Area and Severity Index was evaluated in psoriatic arthritis patients with baseline body surface area â¥3% .
If You Take More Than The Recommended Amount Of Remicade
Call your doctor right away if you believe youve received too much Remicade. Another option is to call the American Association of Poison Control Centers at 800-222-1222 or use its online tool. If you have severe symptoms, immediately call 911 or your local emergency number, or go to the nearest emergency room.
Read Also: Is Green Tea Good For Ulcers
Assessment Of Dose Escalation
Dose escalation was defined differently for each biologic therapy, although the principle was the same. First, an induction period was calculated for each therapy, based on the labeled induction length, with an extra 1 week added for adalimumab and golimumab, and an extra 2 weeks added for infliximab and vedolizumab, to account for slight delays or variation in administration. Induction doses for adalimumab , golimumab , and vedolizumab were ignored for the purpose of calculating dose escalation . If the average dose during maintenance was at least 20% higher than the product label, this was considered dose escalation. For infliximab, since dosing is weight-based and weight was not available from the claims data, the index dose was used to derive an average daily baseline maintenance dose. This was calculated by dividing the total quantity administered at index by 56 . If this estimated average dose during maintenance was at least 20% higher than the index dose, this was considered dose escalation. For all medications, dose escalation was evaluated as the only persistent portion of the maintenance period because the gap might signal a treatment interruption.
Tell Your Doctor Or Ibd Team Immediately If You Develop
- Symptoms that may mean you are having a reaction to the injection or an allergic reaction:
- Hives or other skin rashes
- Trouble breathing or swallowing, or shortness of breath
- Pains in your chest or muscles or joints
- Fever or chills
- Swelling of your face, hands or feet
- Headaches or a sore throat
Living with Crohn’s Disease
Infliximab is often taken alongside other medicines safely. See the earlier section Taking infliximab with other Crohns or Colitis treatments.
However, infliximab may interact with other medicines. Speak to your doctor or pharmacist if youre taking, or plan to take any other medicines. This includes over the counter medicines and any herbal, complementary, or alternative medicines or therapies.
Do not take medicines that contain anakinra or abatacept. These medicines are commonly used for Rheumatoid Arthritis.
Recommended Reading: What Kind Of Yogurt Is Good For Ulcers
Is Remicade A Form Of Chemotherapy
No, Remicade isnt a form of chemotherapy. Remicade is a biologic, which means its made from living organisms. Specifically, Remicade is monoclonal antibody thats made from immune system cells in a lab. Monoclonal antibodies only block the activity of certain proteins in the body.
Chemotherapy, on the other hand, is a chemical drug that destroys rapidly growing cells throughout the body. Its typically used to treat cancer. Chemotherapy medications affect many types of cells and organs. This is different from the very specific actions of monoclonal antibodies, such as Remicade.
Demographic And Clinical Characteristics
Demographic and clinical characteristics at index , were collected. The 25 most common medications prescribed during the 12-month pre-index period were obtained, and those related to 5-ASA, steroids, or immunosuppressants are reported. Immunosuppressant use in the post-index period is also reported.
Recommended Reading: How Serious Is A Stomach Ulcer
Side Effects In Children
In clinical studies, children with Crohns disease or ulcerative colitis had certain side effects more often than adults.
Side effects that occurred more often in children were:
- anemia
- low level of white blood cells
- flushing
- bacterial and viral infections
- allergic reactions of the throat and lungs
Your doctor will monitor your child for these side effects during and after Remicade treatment.
Search Strategy And Study Selection
We searched MEDLINE , EMBASE and CENTRAL databases for records from 2000 to 2016, without language restriction. The following search strategy was used: 1. severe*.mp 2. .mp 3. 1 and 2 4. colitis.ti 5. infliximab.ti 6. 3 and 4 and 5. Bibliographies of relevant studies, review articles and meta-analyses were hand-searched to identify additional studies. This search yielded a total of 400 citations of which 66 were identified as duplicates and removed . Randomised controlled trials , cohort, casecontrol and cross-sectional studies were eligible provided they reported on pharmacokinetics of infliximab in adult acute severe UC patients, on prognostic markers for acute severe UC outcome, or on the use of infliximab treatment intensification strategies in acute severe UC patients. Seventy-six citations were eligible for analysis .
Flow diagram. The search strategy identified 400 references of which 66 were duplicates and removed. Two hundred sixty-four of the remaining 334 records were excluded because they did not meet the eligibility criteria or provided insufficient information for analysis. Six supplemental references were manually retrieved, resulting in a total of 76 records included for analysis.
Also Check: How Do You Treat A Peptic Ulcer
Who Are Our Experts
Pharmacists or Nurses who are specifically trained on all aspects of the Janssen products and the therapeutic areas they treat.
All third party trademarks used herein are trademarks of their respective owners.
This information is intended for healthcare providers in the United States only.This page was last modified on January 02, 2022.
Can Remicade Cause Cancer
Its not clear whether Remicade causes cancer.
There have been reports of new or unusual cancers* with the use of Remicade and other drugs in the tumor necrosis factor-alpha blockers class.
Some of the cancers included lymphoma , skin cancer, and cervical cancer. Many of the cases occurred in younger males, with the exception of cervical cancer.
However, in a review of multiple studies, the evidence for cancer risk was conflicting. Analyses of studies and registries that collect information from larger populations have also had conflicting results.
If you have concerns about cancer, talk with your doctor about your medical history and your risk for cancer. Theyll explain the benefits and risks of taking Remicade. For more information about Remicade and cancer, see the Remicade side effects section above.
for cancer. A boxed warning is the most serious warning from the Food and Drug Administration . For more information, see FDA warnings at the beginning of this article.
Read Also: Does Turmeric Help With Ulcerative Colitis
Whats The Dosage Of Remicade For Children
Remicade is prescribed to treat ulcerative colitis and Crohns disease in children ages 6 years and older. The dosage of Remicade for children is the same dosage thats used for these conditions in adults. For more information, see Dosage for psoriatic arthritis, Crohns disease, plaque psoriasis, and ulcerative colitis above.
Express Ii Study Design16
EXPRESS II evaluated 835 patients with moderate to severe plaque psoriasis. These patients had â¥10% body surface area involvement, a Psoriasis Area and Severity Index score of â¥12, and were candidates for systemic therapy or phototherapy. Patients were randomized to placebo or REMICADE® at doses of 3 mg/kg IV or 5 mg/kg IV at Weeks 0, 2, and 6 . At Week 14, patients in the REMICADE® arms continued on their original dose but were randomized to either an every 8 week scheduled maintenance therapy or an as needed maintenance therapy through Week 46. Patients randomized to the PRN maintenance therapy group received their original REMICADE® dose when baseline improvement in PASI was < 75% and received placebo if PASI improvement was â¥75%. Maintenance visits occurred monthly. At Week 16, the placebo group crossed over to REMICADE® induction therapy , followed by a maintenance therapy every 8 weeks. The primary endpoint was proportion of patients achieving PASI 75 at Week 10.
Recommended Reading: What To Do When Ulcerative Colitis Flares
Dosage In Adult Crohn’s Disease
The recommended dosage of REMICADE is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of adults with moderately to severely active CD or fistulizing CD. For adult patients who respond and then lose their response, consideration may be given to treatment with 10 mg/kg every 8 weeks. Patients who do not respond by Week 14 are unlikely to respond with continued dosing and consideration should be given to discontinue REMICADE in these patients.
Rated For Rheumatoid Arthritis Report
I have been on Remicade for a year and a half with good results. I do have side effects. Within twenty four hours after my treatment, my body starts to ache in every muscle and joint I have. I walk like a baby taking its first steps. All I can do is take a pain and muscle relaxer and go to bed. On the second day after the injection I feel ninety five percent human again. Im able to do gardening and swim again. I did have some hair loss so I take a multiple vitamin and that helps. Since I take Methotrexate, my doctor added folic acid , on pill daily to help with loosing hair and it has. Ive read the revues with the terrible results from using and I feel bad. Remicade is not for everyone. Even I have a bad reaction after infusion. For me it works, and I hope the manufacturer keeps working to make it more effective for all of us without bad side effects.
Read Also: Home Remedies For Oral Ulcers
Outcomes For Acute Severe Uc Patients Receiving Infliximab Rescue Therapy
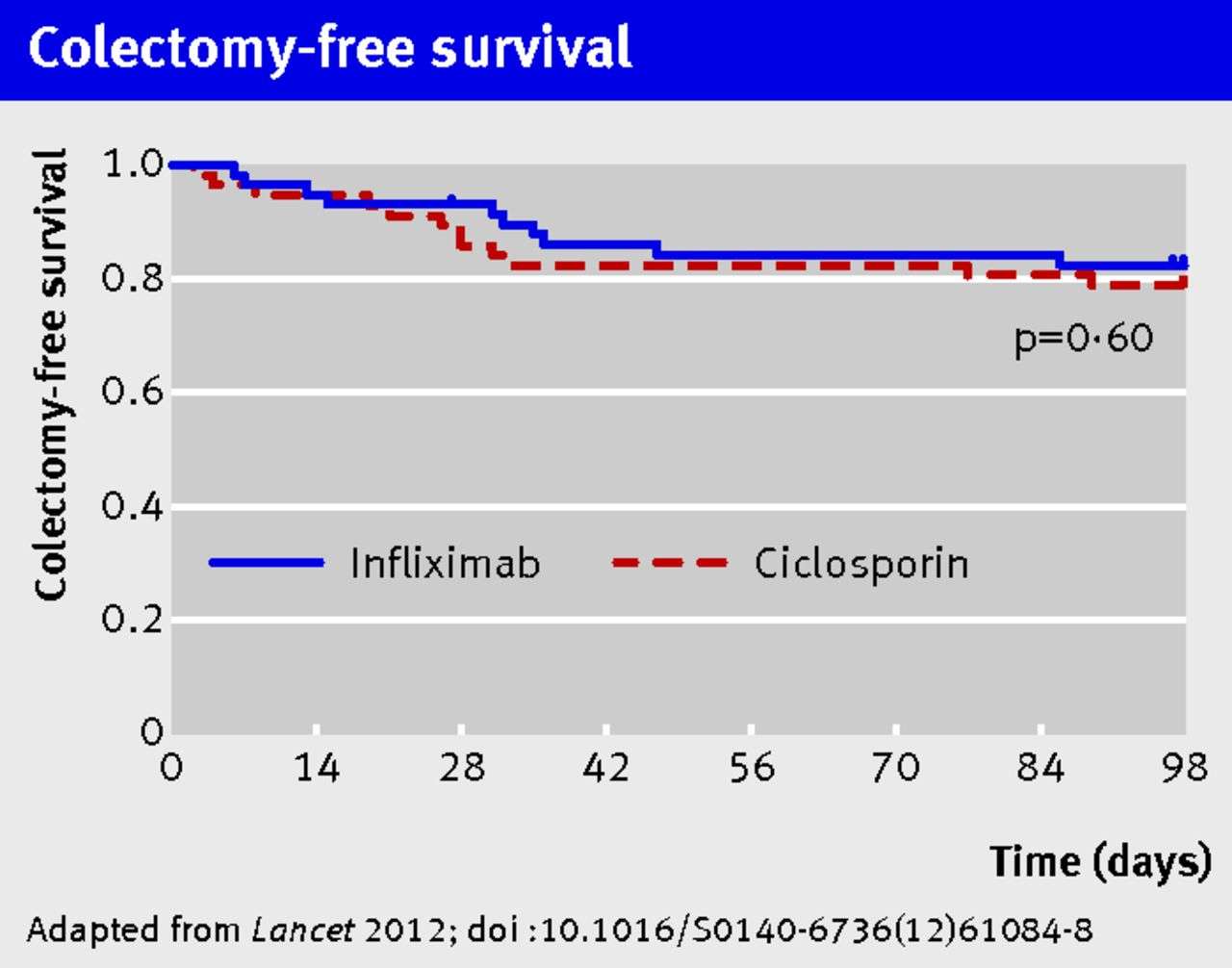
The first randomised controlled trial of infliximab therapy in corticosteroid-refractory acute severe UC was conducted by Sands et al. in 2001 who reported superior clinical, biochemical and endoscopic responses to infliximab compared to placebo. However, the small sample size precluded any definitive conclusions. A subsequent placebo-controlled trial by Jarnerot et al. found a lower 90-day colectomy post-randomisation in moderate-to-severe corticosteroid-refractory UC patients given a single 5 mg/kg infliximab infusion compared with those assigned to placebo . A recent comprehensive review that summarised the efficacy data of infliximab for acute severe UC from both observational and randomised studies reported short-term response rates ranging from 50% to 83%, and long-term rates ranging from 50% to 65%. Short-term colectomy rates ranged from 0% to 50% and long-term rates ranged from 35% to 50% in the infliximab groups.
Based on data from these RCTs, current guidelines do not recommend one option over the other,- although the 2012 Canadian guidelines state that ciclosporin should only be given to azathioprine-naive patients. In practice, however, many gastroenterologists consider infliximab the preferred agent on the basis of ease of administration and lack of nephrotoxicity.