Turning To Remicade For My Ulcerative Colitis
Years ago, I turned to Remicade when I felt I had exhausted all other treatments for ulcerative colitis.
If youre not familiar, Remicade is a prescription medication used to treat adults living with Crohns disease, different forms of arthritis, and UC. This medication belongs to a class of biologics known as tumor necrosis factor alpha inhibitors. Put simply: this medicine administered through IV therapy helps reduce inflammation.
When the FDA approved it for UC and it was available in my home region, I told my then-gastroenterologist, “Sign me up!” That was sometime around 2009-2010.
A Multicenter Retrospective Cohort
The study cohort included 213 patients who were hospitalized with steroid-refractory acute severe UC and received infliximab rescue therapy at one of three centers between 2005 and 2017. The average patient age was 31 and 60% were men.
The researchers divided the patients into two groups according to infliximab administration schedule: standard or accelerated . At two centers, the choice of schedule was based on symptoms or endoscopic severity at the other, it was based on the ratio of C-reactive protein to albumin.
Report A Janssen Product Adverse Event
You may contact the Medical Information Center by calling 1-800-JANSSEN to speak to a clinical expert regarding your question or to report a possible adverse event.
Report a Janssen COVID-19 Vaccine Adverse Event
Report a Adverse Event to FDA VAERS at www.vaers.hhs.gov/reportevent.html or call the FDA at 1-800-822-7967.Report a Adverse Event to Janssen at 1-800-565-4008 or 1-908-455-8822 .
Also Check: Can Ulcerative Colitis Cause Chest Pain
Administration Instructions Regarding Infusion Reactions:
Prior to treatment, ensure appropriate personnel and medication are available to treat reactions that occur during infusion and shortly after infusion. Prior to infusion with Infliximab, patients may be premedicated with histamine-1 receptor antagonists, histamine-2 receptor antagonists, acetaminophen, and/or corticosteroids.
For mild to moderate reactions during the infusion, consider slowing or stopping the infusion. Upon resolution of these reactions, may reinitiate at a lower infusion rate and/or with histamine-1 receptor antagonists, histamine-2 receptor antagonists, acetaminophen, and/or corticosteroids. Discontinue the infusion if the mild to moderate reactions reoccur.
Discontinue the infusion if severe hypersensitivity reactions occur during the infusion.
Reference:1. Infliximab . Horsham, PA: Janssen Biotech, Inc.
SERIOUS INFECTIONS
Patients treated with either REMICADE® or Infliximab are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids. Discontinue either REMICADE® or Infliximab if a patient develops a serious infection or sepsis.
Reported infections include:
MALIGNANCIES
CONTRAINDICATIONS
HEPATITIS B REACTIVATION
HEPATOTOXICITY
HEART FAILURE
HEMATOLOGIC EVENTS
HYPERSENSITIVITY
CARDIOVASCULAR AND CEREBROVASCULAR REACTIONS DURING AND AFTER INFUSION
NEUROLOGIC EVENTS
AUTOIMMUNITY
cp-253861v1
Key Points About Humira
- Humira is approved for both Crohns disease and ulcerative colitis.
- Humira is given at home by self-injection.
- Humira is started with 4 injections, followed by 2 injections 2 weeks later, and then 1 injection every other week.
- Common side effects include pain or irritation at the injection site and headache, rash, and nausea.
- If you are pregnant or plan to become pregnant, you and your doctor should decide if you should take Humira.
- Infants born to mothers receiving Humira should not receive live vaccines for six months.
- Humira needs to be refrigerated.
Don’t Miss: Azulfidine Dosage For Ulcerative Colitis
Administration Instructions Regarding Infusion Reactions
Prior to treatment, ensure appropriate personnel and medication are available to treat reactions that occur during infusion and shortly after infusion. Prior to infusion with REMICADE, patients may be premedicated with histamine-1 receptor antagonists, histamine-2 receptor antagonists, acetaminophen, and/or corticosteroids .
For mild to moderate reactions during the infusion, consider slowing or stopping the infusion. Upon resolution of these reactions, may reinitiate at a lower infusion rate and/or with histamine-1 receptor antagonists, histamine-2 receptor antagonists, acetaminophen, and/or corticosteroids. Discontinue the infusion if the mild to moderate reactions reoccur.
Discontinue the infusion if severe hypersensitivity reactions occur during the infusion.
Recommended Dose And Dosage Adjustment
For recommended intravenous infusion duration for patients with each of the indications described below, see DOSAGE AND ADMINISTRATION, Administration.
Rheumatoid ArthritisThe recommended dose of INFLECTRA® is 3 mg/kg given as an intravenous infusion followed by additional 3 mg/kg doses at 2 and 6 weeks after the first infusion then every 8 weeks thereafter. INFLECTRA®should be given in combination with methotrexate. For patients who have an incomplete response, consideration may be given to adjusting the dose up to 10 mg/kg and/or treating as often as every 4 weeks. Duration of treatment needed to achieve a response after dose escalation is not known. However, higher doses of INFLECTRA®were associated with a slightly higher proportion of patients experiencing adverse events , including infections .
Ankylosing SpondylitisThe recommended dose of INFLECTRA®is 5 mg/kg given as an intravenous infusion followed by additional 5 mg/kg doses at 2 and 6 weeks after the first infusion, then every 6 to 8 weeks thereafter.
PediatricThe recommended dose of INFLECTRA® for pediatric patients with moderately to severely active Crohns disease is 5 mg/kg given as an induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks. Patients who do not respond by week 14 are unlikely to respond with continued dosing and consideration should be given to discontinue INFLECTRA® in these patients.
Special Populations
Renal Impairment
Hepatic Impairment
Don’t Miss: Best Cure For Stomach Ulcer
Remicade Treats Ulcerative Colitis
Better Results Than With Fake Drug, Researchers Report
Dec. 7, 2005 The rheumatoid arthritis drug Remicade may help treat moderate-to-severe ulcerative colitis, a new study shows.
WebMD first reported the news in May, when findings were presented at a medical conference. Now, more details appear in The New England Journal of Medicine.
The researchers included Paul Rutgeerts, MD. He works in Leuven, Belgium, at the University Hospital Gasthuisberg.
Ulcerative colitis is an inflammatory bowel disease that primarily affects the colon and rectum with inflammation and ulcers leading to bleeding and abdominal pain. The disease generally follows a course of flare-ups that can be difficult to manage. In some circumstances, surgery may be necessary to remove the affected area.
You May Like: Remicade Infusion For Ulcerative Colitis
Early Serum Infliximab Levels In Pediatric Ulcerative Colitis
- 1Department of Pediatrics, University of Calgary, Calgary, AB, Canada
- 2Department of Community Health Sciences, University of Calgary, Calgary, AB, Canada
- 3Department of Pediatrics, University of British Columbia, Vancouver, BC, Canada
- 4Department of Pediatrics, University of Manitoba, Winnipeg, MB, Canada
- 5Department of Pediatrics, University of Alberta, Edmonton, AB, Canada
- 6Department of Medicine, University of Calgary, Calgary, AB, Canada
Background: Data on serum infliximab concentrations during induction in pediatric ulcerative colitis are limited. The study aim is to evaluate the relationship between serum infliximab concentrations during induction and short-term clinical remission in children with ulcerative colitis.
Methods: We carried out a prospective, multi-center cohort study in pediatric patients with ulcerative colitis. Serum infliximab concentrations were collected at peak dose #1, week 1, trough pre-dose #2, and trough pre-dose #3. Infliximab dosing was left to investigator discretion. Clinical remission was defined by pediatric ulcerative colitis activity index < 10 at week 8.
Also Check: Causes Of Bleeding Ulcers In Stomach
Read Also: Things To Avoid If You Have Ulcerative Colitis
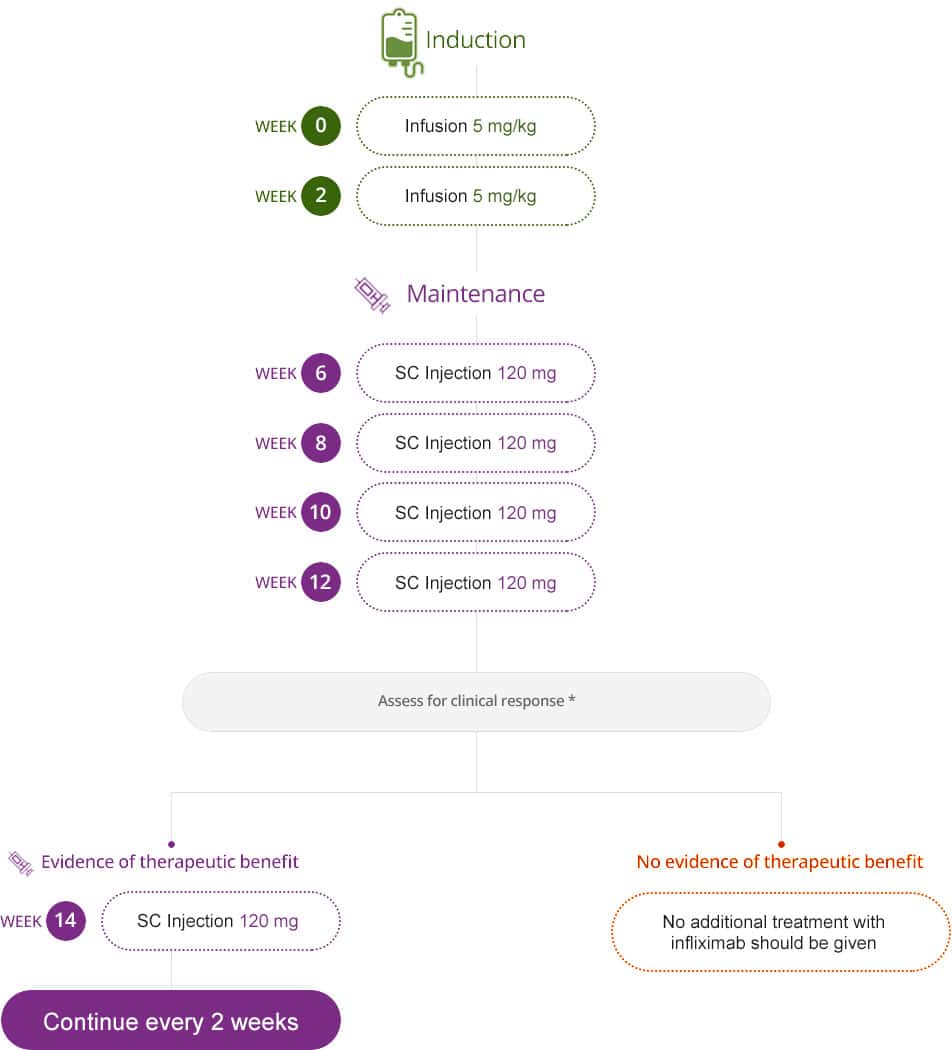
Dosing Of Infliximab For Adults1
Infusions are administered every 8 weeks after 3 induction doses. Infliximab is administered by intravenous infusion over a period of not less than 2 hours.
Moderately to Severely Active Crohnâs Disease*
*Patients who do not respond by Week 14 are unlikely to respond with continued dosing, and consideration should be given to discontinuing Infliximab in these patients.
Moderately to Severely Active Ulcerative Colitis
Moderately to Severely Active Rheumatoid Arthritis
In conjunction with methotrexate.
IICan be used with or without methotrexate.
Chronic Severe Plaque Psoriasis
5 mg/kg 0, 2, and 6 weeks
5 mg/kg 0, 2, and 6 weeks
3 mg/kg 0, 2, and 6 weeks
5 mg/kg 0, 2, and 6 weeks
5 mg/kg 0, 2, and 6 weeks
5 mg/kg 0, 2, and 6 weeks
5 mg/kgevery 8 weeks
For adult patients who respond and then lose their response, consideration may be given to treatment with 10 mg/kg every 8 weeks.
5 mg/kg every 8 weeks
3 mg/kg§every 8 weeks
§For patients who have an incomplete response, consideration may be given to adjusting the dose up to 10 mg/kg every 8 weeks or treating as often as every 4 weeks bearing in mind that risk of serious infections is increased at higher doses per infusion or more frequent dosing.
5 mg/kg every 6 weeks
5 mg/kg every 8 weeks
5 mg/kg every 8 weeks
Tell Your Doctor Or Ibd Team Immediately If You Develop
- Symptoms that may mean you are having a reaction to the injection or an allergic reaction:
- Hives or other skin rashes
- Trouble breathing or swallowing, or shortness of breath
- Pains in your chest or muscles or joints
- Fever or chills
- Swelling of your face, hands or feet
- Headaches or a sore throat
Living with Crohns Disease
Infliximab is often taken alongside other medicines safely. See the earlier section Taking infliximab with other Crohns or Colitis treatments.
However, infliximab may interact with other medicines. Speak to your doctor or pharmacist if youre taking, or plan to take any other medicines. This includes over the counter medicines and any herbal, complementary, or alternative medicines or therapies.
Do not take medicines that contain anakinra or abatacept. These medicines are commonly used for Rheumatoid Arthritis.
Also Check: Nursing Assessment For Ulcerative Colitis
Dosage In Rheumatoid Arthritis
The recommended dosage of REMICADE is 3 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 3 mg/kg every 8 weeks thereafter for the treatment of moderately to severely active RA. REMICADE should be given in combination with methotrexate. For patients who have an incomplete response, consideration may be given to adjusting the dosage up to 10 mg/kg every 8 weeks or treating as often as every 4 weeks bearing in mind that risk of serious infections is increased at higher doses per infusion or more frequent dosing .
Tips For Good Reviews

- Only rate drugs or treatments youve tried.
- In your description, mention the brand, dose, and period of time that you used the drug or treatment.
- Please share your positive and negative experiences with the drug, and compare it with other treatments you have used.
- Do not include any personal information or links in your review.
You May Like: Home Remedies For Skin Ulcers
Side Effects And Risks
Remicade and Humira work in different ways but have some similar side effects. Examples of common and serious side effects for each drug are listed below.
People in both Remicade clinical studies and Humira clinical studies had side effects. But these may be symptoms of other side effects. For example, a fever may be a symptom of an infection. Side effects may overlap between the drugs.
More common side effects
Here are examples of more common side effects that can occur with Remicade, with Humira, or with both drugs .
- Can occur with Remicade:
- psoriatic arthritis
- plaque psoriasis
These drugs havent been directly compared in clinical studies, but studies have found both Remicade and Humira to be effective for treating the conditions mentioned above.
Talking About The Effectiveness Of Medicines
To see how effective a medicine is, we can look at data from clinical trials. Clinical trials are used to test a medicine. However, this data may not completely represent what happens when medicines are given to you by your IBD team. In clinical trials, people are often removed from the trial if they do not respond quickly to a medicine. This wont happen when you start taking infliximab. Your IBD team may advise you take it for a longer time to see if you respond. Theyll also make sure the dose is right for you before suggesting you stop taking it. This means infliximab may be more effective than the data from clinical trials shows.
The best clinical trials include people who were not taking the medicine. This is known as a placebo or control group. This is important. It allows us to see how many people have got better because of the medicine, as well as people who may have got better anyway .
Read Also: L Glutamine Ulcerative Colitis Dosage
Also Check: What Doctor Treats Ulcerative Colitis
Who Are Our Experts
Pharmacists or Nurses who are specifically trained on all aspects of the Janssen products and the therapeutic areas they treat.
©Janssen Scientific Affairs, LLC 2012. All rights reserved.This site is published by Janssen Scientific Affairs, LLC, which is solely responsible for its content.
All third party trademarks used herein are trademarks of their respective owners.
This information is intended for healthcare providers in the United States only.This page was last modified on December 12, 2022.
Reconstitution Dilutionand Administration Instructions1
Infliximab is intended for use under the guidance and supervision of a healthcare provider. The supplied lyophilized powder must be reconstituted and diluted prior to administration. The infusion solution should be prepared and administered by a trained medical professional using aseptic technique by the following procedure:
Calculate the dose, total volume of reconstituted Infliximab solution required, and the number of Infliximab vials needed. More than 1 vial may be needed for a full dose.
Reconstitute each 100-mg Infliximab vial with 10 mL of Sterile Water for Injection, USP, to obtain a concentration of 10 mg/mL, using a syringe equipped with a 21-gauge or smaller needle as follows:
Dilute the total volume of the reconstituted Infliximab solution to 250 mL* with sterile 0.9% Sodium Chloride Injection USP as follows:
- Withdraw a volume from the 0.9% Sodium Chloride Injection, USP, 250-mL bottle or bag equal to the total volume of reconstituted Infliximab required for a dose. Slowly add the total volume of reconstituted Infliximab solution from the vial to the 250-mL infusion bottle or bag
- Discard any unused portion of the reconstituted Infliximab solution remaining in the vial
- Gently invert the bag to mix the solution. The resulting infusion concentration should range between 0.4 mg/mL and 4 mg/mL of Infliximab
Read Also: Mepilex Dressing For Pressure Ulcer
What Are The Typical Dosages Of Remicade
The dosage of Remicade youre prescribed will be based on your weight in kilograms * and the condition youre using Remicade to treat.
Typically, your doctor will start you on the dosage thats recommended to treat your condition. Your doctor may use a dosage calculator to determine this dose. Then theyll monitor your condition over time to make sure the drug is working for you. Your doctor will ultimately prescribe the smallest maintenance dosage that provides the desired effect.
The information below describes dosages that are commonly used or recommended. Your doctor will determine the best dosage to fit your needs.
* One kg is about 2.2 pounds .
Dosage for psoriatic arthritis, Crohns disease, plaque psoriasis, and ulcerative colitis
The recommended dosage level for Remicade is the same when used to treat the following conditions in adults:
For each of these conditions, your starting dose of Remicade is 5 mg per kg of body weight. This is given for your first dose and the doses youll receive at weeks 2 and 6. After that, youll receive 5 mg/kg every 8 weeks.
Over time, Remicade may become less effective at treating Crohns disease in some adults. If this happens, your doctor may increase your Remicade dosage to 10 mg/kg every 8 weeks.
Dosage for ankylosing spondylitis
Dosage for rheumatoid arthritis
Takeaway And Helpful Resources
The dosages in this article are typical dosages provided by the drug manufacturer. If your doctor recommends Remicade for you, they will prescribe the dosage thats right for you. If you have questions about your dosage, talk with your doctor.
Besides learning about dosage, you may want other information about Remicade. These additional articles might be helpful to you:
- More about Remicade. For information about other aspects of Remicade, see this article.
- Side effects. To learn about side effects of Remicade, see this article. You can also look at the Remicade medication guide.
- Details on the conditions Remicade is used to treat, including our lists of:
Recommended Reading: Best Prebiotic For Ulcerative Colitis
Remicade Drug Class And Form
Remicade contains the drug infliximab, which is a biologic . Remicade belongs to a drug class called tumor necrosis factor-alpha blockers. A class of drugs is a group of medications that work in a similar way.
Remicade comes as a vial of powder thats mixed with a liquid solution. The drug is available in one strength: 100 mg.
A healthcare provider will give you Remicade as an infusion. This is an injection into your vein thats given over a period of time. Remicade infusions are typically about 2 hours long. Youll usually receive an infusion every few weeks, but the timing depends on the condition thats being treated.
Recommended Reading: What Causes Mouth Ulcers On Gums
Key Points About Stelara
- Stelara is approved to treat Crohns disease.
- The loading dose of Stelara is given by infusion and thereafter is given by injections at home.
- People taking shots for allergies should talk to their doctor about possible allergic reactions and Stelara.
- Common side effects include infections, injection site reactions, and vomiting.
- If you are pregnant or plan to become pregnant, you and your doctor should decide if you should take Stelara.
- Its thought that Stelara does pass into breastmilk.
- Stelara must be refrigerated.
Recommended Reading: Worst Foods For Stomach Ulcers
Also Check: How Do You Get A Diabetic Foot Ulcer
Effect Of Schedule On Risk Of Colectomy
The proportion of patients who required in-hospital colectomy was similar in the accelerated and standard groups . Neither were there any between-group differences in the longer-term risk of colectomy .
Multivariable analysis confirmed the lack of difference between accelerated and standard infliximab induction with respect to in-hospital and longer-term colectomy rates. Low-serum albumin was the only variable that was consistently an independent predictor of colectomy and it remained significant until almost two years after hospitalization.