Fda Approves Ozanimod For Adults With Ulcerative Colitis
The treatment is also being studied for safety and efficacy in patients with Crohns disease.
The US Food and Drug Administration has approved ozanimod 0.92 mg, an oral agent that selectively targets sphingosine-1-phosphate receptor subtypes 1 and 5, for adult patients with moderately to severely active ulcerative colitis.
The approval, awarded to Bristol Myers Squibb, was based on the data from a placebo-controlled phase 3 trial dubbed True North. In the study, researchers evaluated ozanimod as a single, daily oral therapy for both adults and pediatric patients at least 12 years old with moderately to severely active ulcerative colitis.
Despite the availability of approved therapies, there is still unmet need and an opportunity to deliver additional treatment options to help patients better manage their disease, said Adam Lenkowsky, general manager and head, U.S., Cardiovascular, Immunology and Oncology, Bristol Myers Squibb, in a statement. Were thrilled that our pursuit of transformative science in immunology may benefit patients in their ulcerative colitis treatment by introducing a new option that has a different mechanism of action than available therapies. Zeposia combines disease control through lasting remission and demonstrated safety in a once-daily pill.
Fda Approves Ozanimod As Oral Treatment For Moderately To Severely Active Ulcerative Colitis
The approval represents the first and only oral sphingosine 1-phosphate receptor modulator indicated for adults with moderately to severely active ulcerative colitis.
Officials with the FDA have approved ozanimod as the first and only oral treatment indicated for adults with moderately to severely active ulcerative colitis.
Ozanimod is an oral sphingosine 1-phosphate receptor modulator, which could present a new treatment method for ulcerative colitis, according to a press release from Bristol Myers Squibb. The mechanism of action for ozanimod is unknown, although investigators believe that it may work by reducing lymphocyte migration into the intestines. By targeting the S1P receptors on lymphocytes, the drug subsequently reduces the number of lymphocytes in peripheral blood, according to the release.
Ulcerative colitis can be debilitating and unpredictable for the people living with this chronic inflammatory bowel disease, said Michael Osso, president and CEO of the Crohns and Colitis Foundation, in a press release. The approval of this new oral treatment is welcome news for our community and provides hope to many patients who are looking for new options to achieve symptom relief and remission.
During induction at week 10, the trial met its primary endpoint of clinical remission as well as key secondary endpoints including clinical response , endoscopic improvement , and endoscopic-histologic mucosal improvement .
REFERENCE
How Should I Take Ozanimod
Take ozanimod exactly as prescribed by your doctor. Follow all directions on your prescription label and read all medication guides or instruction sheets.
Ozanimod can slow your heart rate when you start taking it. Before your first dose, your heart function will be checked using an electrocardiograph or ECG .
Ozanimod comes in a 7-day starter pack containing tablets of different colors and strengths. Taking the tablets in order will increase your dose gradually during the first week.
You may take ozanimod with or without food. Avoid foods high in tyramine . Eating these foods while taking this medicine can raise your blood pressure.
You may get infections more easily, even serious or fatal infections. You will need frequent medical tests, and your risk of infection could last for 3 months after you stop taking this medicine.
If you get an infection, further doses may be delayed until your infection clears up.
If you stop taking ozanimod or miss a dose during the first 2 weeks, ask your doctor before you start taking the medicine again. You may need to use a starter pack again, to gradually increase your dose.
Store at room temperature away from moisture and heat.
Always ask your doctor before you stop taking ozanimod for any reason. Your MS symptoms may return and become worse than before or during treatment with this medicine. Tell your doctor if you have any signs of worsening MS.
You May Like: How To Treat Venous Stasis Ulcers
Therapeutic Administration Of Rpc1063 Inhibits Clinical And Histological Disease Scores In Ibd Models
To determine the therapeutic benefit of RPC1063 in other inflammatory conditions we assessed the efficacy of RPC1063 in two preclinical models of inflammatory bowel disease: TNBSinduced colitis in Sprague Dawley rats and naïve T cell adoptive transfer into Severe Combined Immunodeficiency mice.
Colitis was induced by rectal administration of TNBS into Sprague Dawley rats. A dose range of RPC1063 was administered once daily for 7 days in therapeutic mode with the first dose 2 h after TNBS administration. Two rats in the TNBS vehicletreated group died from intestinal rupture on day 6 and 7 of the study in line with the acute nature of this disease model. However, none of the RPC1063 or prednisolonetreated animals died, consistent with improved intestinal health. TNBS vehicletreated mice showed an 18 ± 5% decrease in body weight at the end of the study and did not produce faecal pellets indicating loss of colon function. RPC1063 reduced weight loss in a dosedependent manner, achieving statistical significance for the 1 mg·kg1 group , and restored colon function as demonstrated by faecal pellets in the cage. RPC1063 also reduced the colon weight:length ratio, a surrogate measure of colon inflammation, with the mid and high dose cohorts demonstrating similar efficacy to prednisolone . The overall macroscopic colon disease score improved in a RPC1063 dosedependent manner . Finally, the reduction in circulating lymphocytes correlated with the degree of efficacy .
A New Indication Of Success: Fda Approves Ozanimod For Ulcerative Colitis
The novel drug created at Scripps Research has achieved a second FDA approval, this time for ulcerative colitis, as clinical trials continue for Crohns disease.
May 27, 2021
LA JOLLA, CAOzanimod, the drug invented at Scripps Research that won FDA approval last year for relapsing forms of multiple sclerosis, has been approved in the United States for a second high-need medical condition, ulcerative colitis.
The once-daily oral drug, sold by Bristol Myers Squibb under the name Zeposia, can now be prescribed to treat adults with moderate to severe forms of the inflammatory bowel disease. Notably, its the first drug in a novel class of immune-modulating compounds to be approved for ulcerative colitis, which affects about 1 million people in the United States.
For patients with ulcerative colitis, this oral drug offers a better and more convenient option to control disease progression and improve quality of life, says Hugh Rosen, MD, PhD, who invented ozanimod along with fellow Scripps Research professor Edward Roberts, PhD, and their laboratory colleagues. The hope is that this will lead to fewer dangerous complications or serious infections than current treatment options, providing a steadier path for newly diagnosed patients as well as those failing other treatments.
Additional molecules developed by Rosen and Roberts at Scripps Research are currently in phase 2 clinical trials for major depressive disease and anxiety, and phase 1 studies for treatment of autism.
You May Like: Remicade Dosing For Ulcerative Colitis
E Targeting Leukocyte Circulation Outside The Intestine
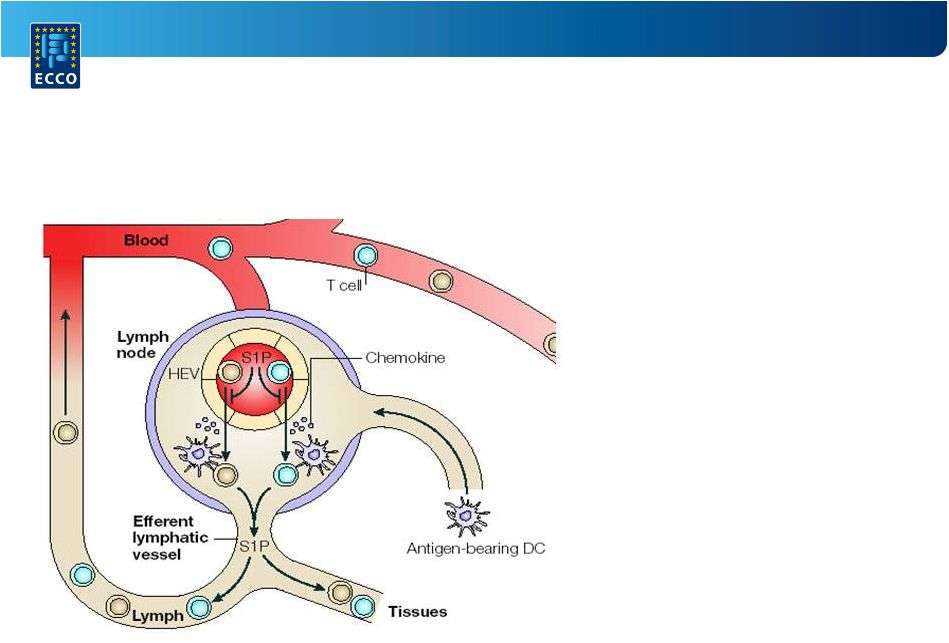
Blockade of lymphocyte recirculation in lymph nodes can be targeted through modulation of sphingosine 1-phosphate receptors. Sphingosine 1-phosphate is a bioactive lysophospholipid metabolite that can act as an intercellular lipid mediator of inflammation. Interaction between S1P and the five known S1P receptors regulate the response and function of biological processes, including cell differentiation, migration, proliferation and trafficking of T and B cells.
Fingolimod was the first S1P receptor modulator to reach the market, initially approved for the treatment of MS . The drug is phosphorylated in vivo, acquiring a structure similar to that of S1P, and functions as a non-selective small-molecule agonist to four of the five S1P receptors. Fingolimod causes peripheral blood lymphopenia due to sequestration of lymphocytes within lymphoid tissue and is associated with cardiovascular side effects, such as bradycardia and hypotension. This led to the development of more specific S1P receptor modulators with preferential actions on S1PR1, S1PR4 and S1PR5, since fingolimod also showed beneficial effects in murine models of colitis .
Carolyn Goldschmidt DO, Marisa P. McGinley DO, MSc, in, 2021
Clinical Trials On Zeposia
The safety and efficacy of ZEPOSIA were demonstrated in two randomised, double-blind, double-dummy, parallel-group, active comparator-controlled clinical trials, Phase 3 SUNBEAM and RADIANCE Part B trials.
In SUNBEAM, 1346 patients were randomised to receive 0.92mg of oral ZEPOSIA which is almost equivalent to 1mg against AVONEX® intramuscular injection weekly during the 12-month treatment period. In RADIANCE Part B, 1,320 patients were treated for 24 months.
The primary endpoint for SUNBEAM and RADIANCE Part B was annualised relapse rates during the treatment periods of 12 and 24 months respectively.
The secondary MRI endpoints in SUNBEAM and RADIANCE Part B enclosed the number of new or enlarging hyper-intense T2-weighted brain MRI lesions over 24 months and the number of gadolinium-enhanced brain MRI lesions at month 24.
The most common adverse reactions observed in the patients during these trials were orthostatic hypotension, urinary tract infection, upper respiratory infection, back pain, elevation in hepatic transaminase and hypertension.
Don’t Miss: Is Garlic Good For Ulcerative Colitis
What Should I Avoid While Taking Ozanimod
Avoid getting up too fast from a sitting or lying position, or you may feel dizzy.
Avoid getting a vaccine without first asking your doctor. While you are taking ozanimod, a “live” vaccine may not fully protect you from disease and you could develop an infection.
Live vaccines include measles, mumps, rubella , rotavirus, typhoid, yellow fever, varicella , and zoster .
You should not receive a live vaccine within 1 month before taking ozanimod, while taking it, and for at least 3 months after you stop taking it.
Pk Of Ozanimod With Gemfibrozil Itraconazole Or Rifampin
This phase 1, randomized, open-label study focused on assessing the single-dose pharmacokinetics of ozanimod and its metabolites as well as to assess the effects of gemfibrozil, itraconazole, and rifampin on the single-dose PK of ozanimod. A total of 40 patients were randomized to receive either a single oral dose of ozanimod, oral doses of gemfibrozil + a single dose of ozanimod, oral doses of itraconazole + a single dose of ozanimod, or oral doses of rifampin + a single dose of ozanimod. In the single dose of ozanimod alone group, there were dose-proportional increases in Cmax and AUC for both the parent drug, ozanimod as well as its metabolites CC112273 and CC1084037. Itraconazole, a strong inhibitor of CYP3A and P-glycoprotein increased ozanimod AUC by 13%, while rifampin, a strong inducer of CYP3A and P-gp, reduced the AUC of ozanimod by 24%. This implies that there is a CYP3A4 and P-gp involvement in the metabolism of ozanimod. Gemfibrozil, a strong inhibitor of the CYP450 system, increased the AUC for the metabolites of ozanimod, CC112273 and CC1084037 by 47% and 69%, respectively. The metabolites of ozanimod were found to have similar single-dose PK properties, with CYP2C8 being the main enzyme in the metabolism of these metabolites, and CYP3A4 and P-gp being enzymes for the metabolism of ozanimod .
L.R. Fitzpatrick, T. Woldemariam, in, 2017
Also Check: Can I Take Tylenol With An Ulcer
Key Tips For A Successful Design Build Project
Thank you.Please check your email to download the eBook.
ZEPOSIA is also an effective treatment of clinically isolated syndrome , relapsing-remitting disease, and active secondary progressive multiple sclerosis, developed by Celgene, a subsidiary of Bristol Myers Squibb . The US Food and Drug Administration approved Ozanimod in March 2020.
The company submitted Marketing Authorization Application for ZEPOSIA for the treatment of relapsing-remitting multiple sclerosis to the European Medicines Agency in March 2019. A regulatory decision from the EMA is anticipated in the second quarter of 2020.
ZEPOSIA is available as an oral capsule with the recommended maintenance dosage of 0.92mg once daily.
Ozanimod In Ulcerative Colitis
Miguel Regueiro, MD: In this section, were going to focus on S1P receptor modulators and other emerging treatments for ulcerative colitis . Ellen, were going to focus on ozanimod now, can you review some of the data from the phase 3 True North study with ozanimod? Give us the overview and any other information you want to add about ozanimod, and then Dave Ill move to you, and then Marla as well. Ellen, why dont you kick us off?
Miguel Regueiro, MD: Nice overview on ozanimod, this new mechanism of action, first in its class showing that there is benefit in the moderate to severe UC study. Nice job outlining the overall data related to True North and some of the more recent data on rapidity of onset with ozanimod, and now long-term extension. Im going to ask all 3 of you this, but Ill start with Dave and then Marla, can you tell me your experience with ozanimod? And Dave, if you want to add anything to what Ellen said about the trials, but I want to get into your experience in your clinics with ozanimod.
Miguel Regueiro, MD: Sometimes beer and wine too, patients ask. And I agree, to put that to rest, the amount of tyramine you would need to have is nearly impossible. I guess nothings impossible in eating. To Daves point about SSRIs, SNRIs, and showing safety, its the same thing with tyramine, its essentially a nonissue. Ellen, Im going to ask you the same question, how are you using ozanimod in your practice?
Also Check: Sacral Dressings To Prevent Pressure Ulcers
Fda Approves Zeposia For Moderate
We were unable to process your request. Please try again later. If you continue to have this issue please contact .
The FDA has approved Zeposia 0.92 mg for the treatment of moderate-to-severe ulcerative colitis.
Zeposia , the first sphingosine 1-phosphate receptor modulator approved for moderate-to-severe active UC, may reduce the lymphocyte migration into the intestines, according to the press release.
The approval is based on results from True North, a phase 3 trial that assessed ozanimod as an induction and maintenance therapy compared with placebo in patients with moderate-to-severe active UC .
At 10 weeks, the trial met its primary endpoint of clinical remission , as well as its secondary endpoints of endoscopic improvement , and endoscopic-histological improvement for ozanimod vs. placebo. During maintenance at 52 weeks , clinical remission was also met , according to the release.
In True North, Zeposia demonstrated efficacy for endpoints such as clinical remission, endoscopic and histological mucosal improvement and safety. All are very relevant considerations for patients with ulcerative colitis,Michael Chiorean, MD, AGAF, FASGE, co-director of IBD Center, Swedish Medical Center in Seattle, Washington, said in the release. Zeposia has the potential to be an important new treatment option for adult patients with moderate to severe ulcerative colitis.
Ozanimod Mechanism Of Action
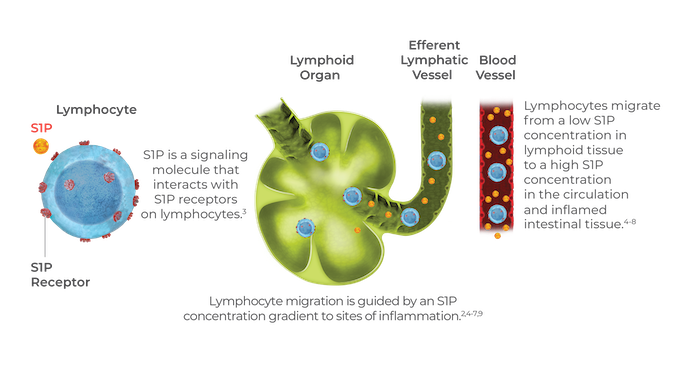
Ozanimod is an oral S1P receptor modulator that targets S1P receptors 1 and 5 with high affinity.
It blocks the ability of lymphocytes to enter the CNS and reduces the number of lymphocytes in the peripheral blood.
BMS is also studying ZEPOSIA as a potential treatment for additional immune-inflammatory indications, such as Crohns disease and ulcerative colitis.
Read Also: Diet If You Have An Ulcer
S1p Receptor Modulator Effective For Both Induction And Maintenance
This article is a collaboration between MedPage Today and:
The oral sphingosine-1-phosphate receptor modulator ozanimod was effective for induction in moderately to severely active ulcerative colitis , meeting the primary endpoint in a phase III trial.
At week 10, clinical remission was achieved by 18.4% of patients randomized to ozanimod 0.92 mg daily compared with 6% of those given placebo, which represented a difference of 12.4% , reported Brian Feagan, MD, of Western University in London, Ontario.
Clinical remission was defined as having the following on the 3-component Mayo score: rectal bleeding score of zero, stool frequency score of 1 or less and decrease from baseline of at least 1, and endoscopy subscore of 1 or less without friability, he explained during a poster presentation at the Advances in Inflammatory Bowel Diseases virtual meeting.
In physiologic conditions, approximately 2% of the total lymphocyte pool is located in the circulation. S1P regulates lymphocyte migration from lymphoid tissue to sites of inflammation. Ozanimod binds to and internalizes the S1P subtype 1 receptor, preventing certain proinflammatory lymphocytes from exiting the lymph nodes and circulating to the intestinal tissue. This mechanism has also been seen to be effective in multiple sclerosis, the original indication for which ozanimod was approved earlier this year.
The current study consisted of a 10-week induction period and a 52-week maintenance phase.
Multiple Sclerosis Causes And Symptoms
Multiple sclerosis is an autoimmune condition that damages the brain and spinal cord, which make up the central nervous system . It disrupts the data flow within the brain and along the nerve pathways between the brain and the body.
The exact cause of multiple sclerosis is unknown and the disease causes the immune system to attack the myelin sheath, resulting in inflammation, scars or lesions on the protective myelin layer around nerve fibres, leading to signal destruction to and from the brain.
Multiple sclerosis is one of the leading causes of non-traumatic neurological disability in young adults.
The unpredictable symptoms of multiple sclerosis are numbness, tingling, mood changes, memory problems, pain, fatigue, and blindness or paralysis. Losses can be temporary or long-lasting.
Multiple sclerosis is categorised into three types including primary progressive MS , secondary progressive MS and relapsing-remitting MS .
Don’t Miss: How To Get Rid Of Mouth Ulcers Overnight
Ozanimod As Therapy For Ulcerative Colitis
- The New England Journal of Medicine
You’ve saved your first item
You can find your saved items on your dashboard, in the “saved” tab.
You’ve recommended your first item
Your recommendations help us improve our content suggestions for you and other PracticeUpdate members.
You’ve subscribed to your first topic alert
What does that mean?