Are Remicade And Humira Safe To Take During Pregnancy Or While Breastfeeding
Researchers havent conducted studies of either of these drugs in pregnant or nursing mothers, though monkey studies of Humira showed no ill effects to the fetuses.
In the absence of research, doctors must weigh the risks and benefits before prescribing Remicade or Humira to pregnant or breastfeeding mothers.
Because of the potential immune suppression effects, however, the prescribing information recommends against administering live vaccines to a baby within six months of being exposed to a TNF inhibitor.
What Is The Safest Biologic For Ulcerative Colitis
The benefits of biologics may outweigh the potential risks in people with moderate-to-severe IBD.
However, biologics can affect how the immune system works. Specifically, they may impact the immune systemâs ability to ward off certain infections.
The risk of infection tends to be higher with anti-TNF agents than with other types of biologics.
The 2020 review of research found that the overall rate of serious infections in people using biologics was low. Entyvio was the safest biologic for treating ulcerative colitis. It was linked to the lowest number of infections.
The authors ranked Stelara as the next safest biologic for treating ulcerative colitis.
Biologics may carry other risks of side effects. A person should speak with a doctor about the potential risks of biologics before they use them.
However, biologics are relatively new medications. For this reason, long-term safety data are limited.
Scientists have completed fewer long-term studies on Entyvio or Stelara. The available data suggest that these biologics are safer than anti-TNF agents.
People with moderate-to-severe ulcerative colitis may need to use biologics on an ongoing basis to keep the condition in remission. Stopping treatment with biologics may cause a relapse, during which symptoms return.
More research is necessary to confirm if and when people can stop using biologics without experiencing a relapse.
Some research has linked biologics to modest weight gain in people with ulcerative colitis.
Key Points About Simponi
- Simponi is approved to treat ulcerative colitis.
- Simponi is given by injection at home.
- Simponi is started with two injections, followed by one injection two weeks later, and one injection every four weeks thereafter.
- Common side effects include pain or irritation at the injection site and upper respiratory or viral infections.
- If you are pregnant or plan to become pregnant, you and your doctor should decide if you should take Simponi.
- It’s not currently known how Simponi will affect a nursing infant.
- Simponi must be refrigerated.
Read Also: Ulcerative Colitis And Stomach Pain
How Effective Are Biologics For Ulcerative Colitis
Biologics can help bring ulcerative colitis into remission. Remission occurs when the symptoms of ulcerative colitis are gone.
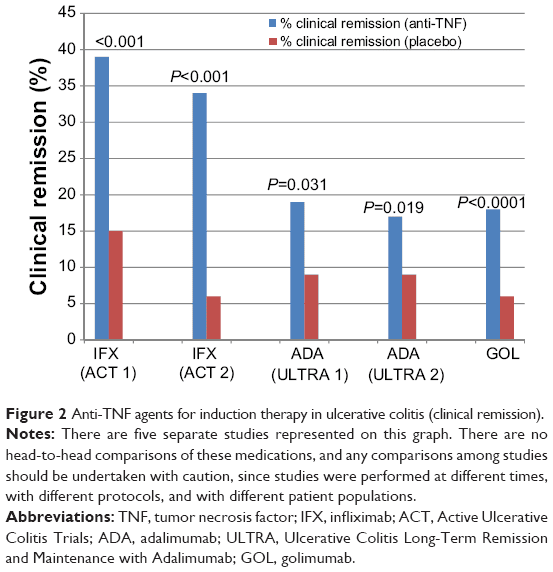
A 2020 review of research found that Remicade was the most effective biologic for reducing the signs and symptoms of ulcerative colitis in people who had never used biologics before.
The same review found that Stelara was the most effective biologic for treating ulcerative colitis in people who had used anti-TNF agents in the past without satisfying results.
Tofacitinib was also highly effective for treating ulcerative colitis in people who had previously used anti-TNF agents. Xeljanz is a JAK inhibitor, which is not a biologic.
These results reflect average trends. Different medications may affect different individuals in different ways. People may need to try multiple medications to find one that works for them.
The authors of one 2019 review reported that up to 30% of people with ulcerative colitis eventually need surgery to treat it. Some evidence has suggested that using biologics may delay the need for surgery.
Which Drugs Interact With Remicade And Humira
Some vaccines contain living bacteria and active viruses to condition the immune reaction. Doctors should never administer these vaccines to people taking either Remicade or Humira because of the infection risk.
Take neither infliximab nor adalimumab if you are taking other drugs, like anakinra , which suppress the immune system as the effect will multiply.
Combination of Remicade and Humira with other, non biologic DMARDs like methotrexate can increase the symptom relief over prescribing either drug alone. Doctors, therefore, frequently prescribe TNF inhibitors and non biologic DMARDs in tandem.
Also Check: How To Treat An Ulcer On Your Tongue
Patients Having Received First
Baseline characteristics
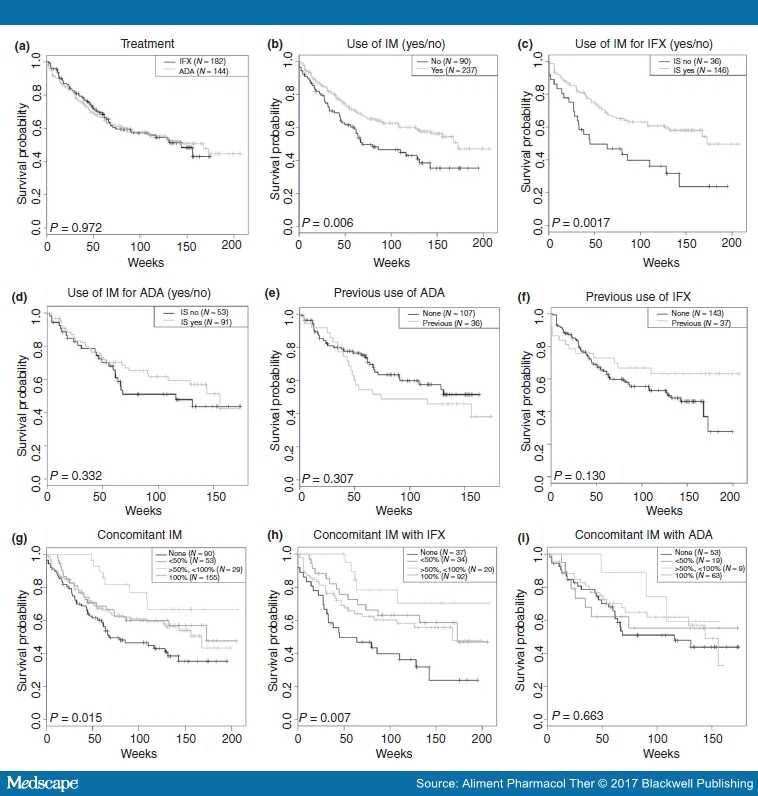
Infliximab was prescribed as first-line anti-TNF treatment in 126 patients , whereas adalimumab was prescribed in 34 patients . The median time interval between disease onset and the initiation of maintenance treatment with infliximab or adalimumab as first-line anti-TNF treatment was 3 years.
The medications taken before the initiation of an anti-TNF agent were variously 5-aminosalicylate , systemic steroids , azathioprine , methotrexate , rectal therapy , cyclosporine , enteral or parenteral nutrition , and investigational drugs .
A total of 130 patients were receiving other treatments on initiation of first-line treatment with an anti-TNF agent: 60 patients were receiving 5-aminosalicylate, 88 patients were receiving systemic steroids, 57 patients were receiving azathioprine, 11 patients were receiving methotrexate, 10 patients were receiving rectal therapy, and 3 patients were receiving enteral or parenteral nutrition.
Detailed data on all treatments before or on initiation of first-line treatment with an anti-TNF agent are given in Supplementary Table S1.
Retention rates and reasons for anti-TNF withdrawal
Persistence of first-line treatment with an anti-TNF agent
The overall mean persistence of first-line treatment with an anti-TNF agent was 3.1 years, with values of 3.4 years for infliximab and 2.1 years for adalimumab. The difference in persistence between the infliximab and adalimumab subgroups was not statistically significant .
Infliximab Versus Adalimumab Which One Is Better For Ulcerative Colitis
Corresponding AuthorEun Soo KimORCIDE-mailCopyrightComparison of Long-term Outcomes of Infliximab versus Adalimumab Treatment in Biologic-Naïve Patients with Ulcerative Colitis
Since the introduction in the mid-2000s, anti-tumor necrosis factor have been considered as paradigm-changing treatment in the management of patients with ulcerative colitis . In the pivotal clinical trials of anti-TNF agents for the management of UC, the rates of clinical remission and clinical response in anti-TNF treated group were significantly higher than in the placebo group during induction and maintenance phase. Use of these agents reduces the risk of poor clinical outcomes including hospitalization,1 cumulative corticosteroid exposure,2 and early phase surgery in patients with UC.3 In addition, they also improve health related quality of life which is an important patient-reported outcome.4
As phenotype of UC is different in various ethnic groups which may be linked to different genetic backgrounds, it is crucial to have data of specific drug efficacy in diverse populations. In line with this notion, the study by Lee et al. is clinically relevant in that it was conducted in Korea where inflammatory bowel disease incidence has been rapidly rising. The real-world study from other Asian countries is warranted to confirm the result of the current study.
You May Like: What Foods Should Be Avoided With Stomach Ulcers
Entyvio Should Be First Choice For Patients With Uc Who Previously Failed Remicade
We were unable to process your request. Please try again later. If you continue to have this issue please contact .
In a direct comparison with Humira, Entyvio won out as the top choice for second line therapy for patients with ulcerative colitis who failed therapy with Remicade, according to research published in Inflammatory Bowel Diseases.
Massimo Claudio Fantini, MD, PhD, from the University of Rome, and colleagues wrote that there are no solid data to support which drug should be used as a follow-up in patients who do not respond or lose response to Remicade .
At the moment, there is no clear indication on the most appropriate second-line biological therapy in case of failure to , and in the absence of head-to-head comparative efficacy data, the choice between a second anti-TNF-alpha or the anti-integrin is often based on clinicians personal experience, drug availability, and economic issues, they wrote.
Researchers analyzed clinical records of 161 patients with UC who failed infliximab therapy and were candidates to receive either Humira or vedolizumab. The primary endpoint was therapeutic failure at week 52, and secondary endpoints included therapy discontinuation at weeks 8, 24 and 52, discontinuation-free survival and safety.
Patients in the study were either primary or secondary infliximab failures or infliximab intolerants . Researchers determined that 64 patients received adalimumab and 97 patients received vedolizumab .
Can I Use Stelara And Humira Together
Most likely not.
In general, biologic disease-modifying antirheumatic drugs , such as Stelara and Humira, shouldnt be used together. Doing so increases the risk of serious side effects from these drugs.
Biologic DMARDs are newer, more targeted treatments that help reduce inflammation by suppressing specific parts of your immune system.
Even though biologic DMARDs arent prescribed together, sometimes doctors prescribe a biologic DMARD with a traditional DMARD. Methotrexate is an example of a traditional DMARD. Traditional DMARDs are older, less targeted drugs used to reduce inflammation in your body.
Also, if your condition isnt controlled with your current biologic DMARD, your doctor may switch your treatment to another biologic DMARD. But this depends on many factors, such as:
- past treatments or other current medications
- any side effects youve experienced
- your overall health
Also Check: Antibiotics And Antiseptics For Venous Leg Ulcers
Stelara And Other Medications Or Therapies
Below are lists of medications and therapies that can interact with Stelara. These lists do not contain all the drugs that may interact with Stelara.
Before taking Stelara, talk with your doctor and pharmacist. Tell them about all prescription, over-the-counter, and other drugs you take. Also tell them about any vitamins, herbs, and supplements you use. Sharing this information can help you avoid potential interactions.
If you have questions about drug interactions that may affect you, ask your doctor or pharmacist.
Stelara and vaccines
You shouldnt get a live vaccine when youre using Stelara. Getting a live vaccine during Stelara treatment increases your risk of getting the condition the vaccine is meant to prevent.
This is because Stelara suppresses your immune systems ability to fight infections. Receiving a live vaccine during Stelara treatment increases your risk of serious infections.
Examples of live vaccines that you should avoid during Stelara treatment include:
You should also avoid getting the Bacillus Calmette-Guérin vaccine for 1 year before you start using Stelara, during your Stelara treatment, and for 1 year after you stop using Stelara. The BCG vaccine is meant to prevent tuberculosis . Its more commonly given to people who live outside of the United States.
Stelara and allergy shots
Stelara and warfarin
If youre taking these drugs together, your doctor may need to adjust your dosage of either Stelara or warfarin.
What Are The Main Differences Between Remicade Vs Humira
Remicade and Humira are both TNF blockers, also known as TNF-alpha blockers. Remicade contains the ingredient infliximab. Remicade is given as an IV infusion by a healthcare professional.
Humira contains the ingredient adalimumab. It is available as a pen injection or a prefilled syringe injection and is given subcutaneously . After proper training, Humira can be self-injected by the patient, or administered by a caregiver.
| Main differences between Remicade and Humira | ||
|---|---|---|
| Remicade | ||
| Varies | ||
| Who typically uses the medication? | Adults and children | Adults and children |
Recommended Reading: Sacral Dressings To Prevent Pressure Ulcers
This Newer Biologic May Be Safer And More Effective Than Older And More Commonly Prescribed Drugs
When the anti-tumor necrosis factor drug infliximab hit the market 20 years ago, it was considered revolutionary for patients with Crohns disease and ulcerative colitis, two forms of inflammatory bowel disease . A handful of other anti-TNF drugs, including adalimumab and etanercept soon followed, and though theyve made a huge difference for many IBD patients they also carry a risk of such side effects as serious infections.
Yet many doctors have been reluctant to switch to vedolizumab , a different type of biologic drug that was FDA approved in 2014.
Were approaching a quarter century of experience with TNF antagonists. TNF antagonists, you could argue, are yesterdays drugs, said Brian G. Feagan, MD, of Western University in London, Ontario, at the annual Advances in Inflammatory Bowel Diseases meeting. So why are so many doctors sticking with them? Unfortunately, gastroenterologists dont change quickly. Were subjects of habit and there have to be compelling reasons for us to change,Healio reported Dr. Feagan as saying during his presentation.
Vedolizumab is an integrin receptor antagonist. Because it only targets the gut, its believed to be safer than the other drug in this class .
Vedolizumab is currently FDA-approved for adults with moderate-to-severe IBD who havent responded well to other older medications, including anti-TNF drugs. But there is evidence to suggest that it might work just as well in patients who have never tried anti-TNF medications.
Drug Interactions Of Remicade Vs Humira
Remicade or Humira should not be combined with other biological products used to treat the same conditions, due to an increase in serious infections and other complications. Examples of interacting drugs include anakinra, abatacept, or tocilizumab.
Remicade or Humira also can interact with drugs that have a narrow therapeutic index. This means a drug has a small window between being safe and effective, and being toxic. These drugs include Coumadin , cyclosporine, and theophylline.
People taking Remicade or Humira should not receive live vaccines.
This is not a full list of drug interactions and other drug interactions may occur. Before taking Remicade or Humira, tell your healthcare provider about all of the medications you take, including Rx and OTC drugs, vitamins, and supplements.
| Drug |
| Yes |
Read Also: Natural Remedies For Duodenal Ulcer
Could Remicade Stop Working For Me
Yes. Remicade may become less effective for you over time. This can occur if your bodys immune system starts to recognize Remicade as a foreign invader and makes antibodies to Remicade. Antibodies are immune system proteins that fight foreign substances, including medications such as Remicade.
If your body makes anti-Remicade antibodies, the drug will clear from your system faster and wont be as effective.
Remicade may also stop working for a time because of stress, dietary choices, or other health conditions.
If youre concerned about how effective Remicade is in treating your condition, talk with your doctor.
Can I Use Remicade Or Humira With Alcohol
Remicade and Humira do not interact with alcohol. However, alcohol can worsen many of the conditions that Remicade or Humira treat, or aggravate the side effects that they may cause. Alcohol can also interact with other medications taken for these conditions. Check with your healthcare provider regarding alcohol use.
Also Check: Is Ulcerative Colitis A Gastrointestinal Disease
Key Points About Stelara
- Stelara is approved to treat Crohn’s disease.
- The loading dose of Stelara is given by infusion and thereafter is given by injections at home.
- People taking shots for allergies should talk to their doctor about possible allergic reactions and Stelara.
- Common side effects include infections, injection site reactions, and vomiting.
- If you are pregnant or plan to become pregnant, you and your doctor should decide if you should take Stelara.
- It’s thought that Stelara does pass into breastmilk.
- Stelara must be refrigerated.
Infusions Every 8 Weeks After 3 Starter Doses
REMICADE® is given as an intravenous infusion by a healthcare professional through a needle placed in a vein in your arm.
Given over a period of about 2 hours
Weeks 0, 2, and 6
After starter doses, 1 maintenance dose is infused every 8 weeks
Your doctor will determine the right dosage of REMICADE® for you.
You May Like: Ulcerative Colitis Lower Back Pain
How Should Remicade Be Taken
- You will be given Remicade through a needle placed in a vein in your arm.
- Your doctor may decide to give you medicine before starting the Remicade infusion to prevent or lessen side effects.
- Only a healthcare professional should prepare the medicine and administer it to you.
- Remicade will be given to you over a period of about 2 hours.
If you have side effects from Remicade, the infusion may need to be adjusted or stopped. In addition, your healthcare professional may decide to treat your symptoms.
A healthcare professional will monitor you during the Remicade infusion and for a period of time afterward for side effects. Your doctor may do certain tests while you are taking Remicade to monitor you for side effects and to see how well you respond to the treatment.
Your doctor will determine the right dose of Remicade for you and how often you should receive it. Make sure to discuss with your doctor when you will receive infusions and to come in for all your infusions and follow-up appointments.
Leaving Abbvie Web Site
You are leaving the AbbVie Web site and connecting to a site that is not under the control of AbbVie. AbbVie is not responsible for the contents of any such site or any further links from such site. AbbVie is providing these links to you only as a convenience and the inclusion of any link does not imply the endorsement of the linked site by AbbVie. You should also be aware that the linked site may be governed by its own set of terms and conditions and privacy policy for which AbbVie has no responsibility.
Conversely, the presence of this link does not imply the linked site’s endorsement of HUMIRA.com or AbbVie.
Do you wish to leave this site?
Don’t Miss: How To Treat An Eye Ulcer
Remicade Vs Humira: Differences Similarities And Which Is Better For You
Drug overview & main differences | Conditions treated | Efficacy | Insurance coverage and cost comparison | Side effects | Drug interactions | Warnings | FAQ
Remicade and Humira are two brand-name medications used for inflammatory bowel disease rheumatoid arthritis, and various other autoimmune diseases. You may have heard of these drugs in the news. Humira is made by AbbVie, and has been the worlds top-selling drug since 2012. Remicade is made by Janssen Biotech, Inc.
Both Remicade and Humira are prescription drugs approved by the U.S. Food and Drug Administration . They are known as biologics and are monoclonal antibodies. Remicade and Humira are in a drug class called tumor necrosis factor blockers, or TNF blockers. They work by blocking the activity of tumor necrosis factor, or TNF. TNF is a substance in the body that can cause inflammation and lead to various diseases related to the immune system. TNF blockers, also called TNF inhibitors, suppress the immune system, blocking the activity of TNF.
Although both Remicade and Humira are both TNF blockers, they do have some differences. Continue reading below to learn about Remicade and Humira.