Safety And Efficacy Trial Of Rpc1063 For Moderate To Severe Ulcerative Colitis
| The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read our disclaimer for details. |
| First Posted : May 6, 2015Results First Posted : September 1, 2021Last Update Posted : September 1, 2021 |
| Phase 3 |
| Study Type : | |
| Quadruple | |
| Primary Purpose: | Treatment |
| Official Title: | A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Trial of Oral RPC1063 as Induction and Maintenance Therapy for Moderate to Severe Ulcerative Colitis |
| Actual Study Start Date : |
| 1mg, daily oral administration during Induction and Maintenance periods. | Drug: RPC1063 |
| Daily oral administration during Induction and Maintenance periods. | Drug: Placebo |
Working On A Manuscript
| Digital Features for this Adis Drug Evaluation can be found at |
| First S1PR modulator approved to treat moderately to severely active ulcerative colitis |
| Significantly improves clinical remission rates relative to placebo as induction and maintenance therapy |
| Generally well tolerated infection-related or cardiovascular AEs are manageable and transient |
Dosage And Administration Of Ozanimod
Oral ozanimod is indicated for the treatment of adults with moderately to severely active ulcerative colitis in the USA , and in adults with moderately to severely active ulcerative colitis who have had an inadequate or lost response to, or were intolerant of, either conventional therapy or a biologic in the EU . Ozanimod should be initiated with a 7-day titration , after which the recommended dosage is 0.92 mg once a day the capsules may be taken with or without food . Patients should be assessed for complete blood count, cardiac and liver function, vaccination status, and current or prior medications before and during ozanimod therapy .
Ozanimod is contraindicated in those with immunodeficiency , severe liver impairment, and a history or presence of second-degree atrioventricular block Type II, third-degree atrioventricular block or sick sinus syndrome, unless the patient has a functioning pacemaker . Ozanimod causes a temporary reduction in peripheral blood lymphocyte count , which may increase susceptibility to infections. Concomitant use with monoamine oxidase inhibitors or CYP2C8 inducers is not recommended. PML has been reported with S1PR modulator use ozanimod therapy should be suspended if PML is suspected, and discontinued if PML is confirmed .
Local prescribing information should be consulted for detailed information regarding further contraindications, dose titration schedules, use in special patient populations, and warnings and precautions.
Don’t Miss: Is Garlic Good For Ulcerative Colitis
Ulcerative Colitis Prescribing Considerations
The FDA granted approval for the use of ozanimod for the treatment of moderate to severely active UC in adults in May of 2021.74 Current prescribing guidelines do not limit therapy for those with prior biologic or immune modulator failure. Given the adverse event profile observed in the ozanimod clinical trials and those for other S1P modulators, several steps are suggested before and during treatment to help mitigate the occurrence and severity of these events.65
Although changes in PFTs were reported in the phase 1 trial, later trials did not report any incidence of pulmonary adverse events. Nevertheless, baseline PFTs should be considered in patients with severe obstructive or restrictive pulmonary disease. Baseline ophthalmologic evaluation is recommended for patients with diabetes mellitus, uveitis, or macular edema. It is also recommended to test all patients for varicella zoster virus antibodies who do not have either a health-care professionalconfirmed history of varicella or without documentation of a full course of vaccination against VZV. If negative, VZV vaccination is recommended at least one month prior to initiating ozanimod therapy.
Ozanimod use is not recommended in patients with active infection or hepatic impairment.
Nice Recommends First Oral S1p Receptor Modulator For Ulcerative Colitis
Posted: 7 October 2022 | Catherine Eckford |
NICE has recommended the drug Zeposia® for moderate to severe cases of ulcerative colitis.
The National Institute of Health and Care Excellence has recommended Bristol Myers Squibbs Zeposia® for use in England and Wales to treat moderate to severe ulcerative colitis cases in adults who are intolerant of, or unresponsive to biologics or conventional therapies.
UC is an Inflammatory Bowel Disease that makes immune system attack the gastrointestinal tract. Symptoms include swelling and inflammation of the rectum and colon. Demographic data reports that the disease mainly affects individuals between 15 to 25 years old. These symptoms can flare-up several times per year, causing patients to need the bathroom multiple times a day, become anaemic and lose their appetite, resulting in unintended weight loss.
One in 227 of the UK population have UC. Half of cases are moderate to severe, so NICEs recommendation has garnered positive welcome from healthcare professionals and patients around the country.
A Phase III trial titled True North, evaluated the efficacy of Zeposia® against placebo to help participants reach either clinical remission or endoscopic improvement and mucosal healing.
Zeposia® reduces the capacity of lymphocytes to emerge from lymph nodes, reducing the number of lymphocytes circulating in the blood.
Also Check: Hiv Ulcers In Mouth Pictures
Clinical Trials: Ulcerative Colitis
Phase Two
The phase two Touchstone trial49 of ozanimod for induction and maintenance therapy for moderate to severe UC was a multi-center, double-blind, placebo-controlled trial consisting of 197 adults with moderate-to-severe UC, determined by a Mayo Clinic score of 612 and endoscopic sub-score of 23. Patients were randomly assigned, in equal ratios, to receive ozanimod at a dose of 0.5 mg or 1 mg or placebo once daily. The trial consisted of induction until week 8, maintenance from week 8 to 32, and an open label extension period after week 32.
The primary outcome of the study was clinical remission, defined as a Mayo Clinic score 2, with no sub-score > 1, at 8 weeks. Secondary outcomes evaluated at week 8 included clinical response , change from baseline Mayo Clinic score, and mucosal healing .
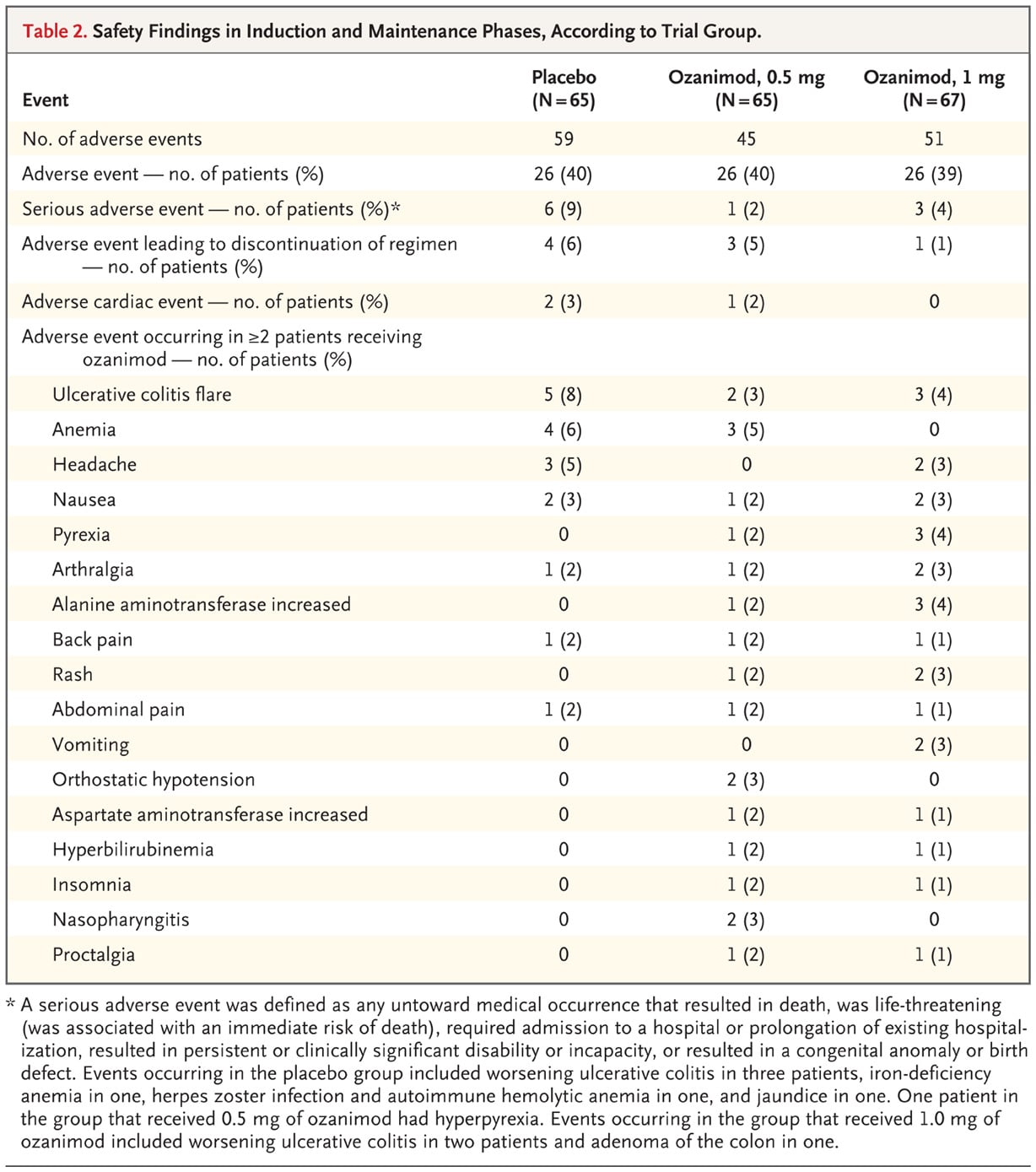
Adverse events were similar in all three study groups. The most common TEAEs were UC flare, anemia, headache, nausea, pyrexia, arthralgia, and elevated ALT which occurred in 4 patients across all three groups. Interestingly, adverse cardiac events occurred in the placebo and 0.5mg ozanimod groups but not in the 1mg ozanimod group. UC flare, pyrexia, and elevated ALT were the most common adverse events in the 1mg ozanimod group with 4% of patients experiencing each adverse outcome.
Discontinuation rates were 28% at year 1 and 1518% annually through year 4 which is superior to reported discontinuation rates for the TNF- inhibitors infliximab and adalimumab.68
Phase Three
Efficacy And Safety Of Combination Induction Therapy With Guselkumab And Golimumab In Participants With Moderately To Severely Active Ulcerative Colitis: Results Through Week 12 Of A Phase 2a Randomized Double
Guselkumab is an antagonist of the p19 subunit of IL-23 that is approved to treat plaque psoriasis. Golimumab is an antagonist of TNF and is approved for the treatment of ulcerative colitis.1 The phase 2a VEGA study evaluated the safety and efficacy of induction therapy with guselkumab plus golimumab vs monotherapy with guselkumab or golimumab in adults with moderately to severely active ulcerative colitis.2The trial enrolled patients with a Mayo score of 6 to 12 and an endoscopy subscore of 2 or lower according to central review. The patients had received prior therapy that was either intolerable or unsuccessful. The trial excluded patients previously treated with a TNF antagonist.
Patients were randomly assigned into the 3 arms. In the golimumab monotherapy arm, this agent was administered at 200 mg subcutaneously at week 0, followed by 100 mg administered at weeks 2, 6, and 10. In the guselkumab monotherapy arm, treatment was administered at 200 mg intravenously at weeks 0, 4, and 8. For combination therapy, the 2 antibodies were administered in combination at the same doses and schedules. The induction phase for all 3 arms continued for 12 weeks. The primary endpoint was clinical response, defined as a decrease from baseline in the Mayo score of at least 30% and 3 points, with either a decrease in the rectal bleeding subscore of 1 or more or a rectal bleeding subscore of 0 or 1.
References
You May Like: Foods That Heal Stomach Ulcers
Fda Approves Ozanimod As Oral Treatment For Moderately To Severely Active Ulcerative Colitis
The approval represents the first and only oral sphingosine 1-phosphate receptor modulator indicated for adults with moderately to severely active ulcerative colitis.
Officials with the FDA have approved ozanimod as the first and only oral treatment indicated for adults with moderately to severely active ulcerative colitis.
Ozanimod is an oral sphingosine 1-phosphate receptor modulator, which could present a new treatment method for ulcerative colitis, according to a press release from Bristol Myers Squibb. The mechanism of action for ozanimod is unknown, although investigators believe that it may work by reducing lymphocyte migration into the intestines. By targeting the S1P receptors on lymphocytes, the drug subsequently reduces the number of lymphocytes in peripheral blood, according to the release.
Ulcerative colitis can be debilitating and unpredictable for the people living with this chronic inflammatory bowel disease, said Michael Osso, president and CEO of the Crohns and Colitis Foundation, in a press release. The approval of this new oral treatment is welcome news for our community and provides hope to many patients who are looking for new options to achieve symptom relief and remission.
During induction at week 10, the trial met its primary endpoint of clinical remission as well as key secondary endpoints including clinical response , endoscopic improvement , and endoscopic-histologic mucosal improvement .
REFERENCE
How Does Ozanimod Measure Up
To see how ozanimod stacks up to the two older drugs, Dubinsky and colleagues weighted the data from True North to match the patient populations in the other trials by age, sex, baseline total Mayo score, disease extent, and prior anti-TNF treatment.
They calculated the odds that ozanimod would produce better clinical and endoscopic responses or be associated with more serious or infectious adverse events in comparison with each of the other drugs. The comparisons included both the induction and maintenance phases of the trials.
The researchers compared ozanimod to adalimumab for patients who were anti-TNF naive. They found that the patients who took ozanimod were more likely to experience a clinical response than those who took adalimumab . The patients who took ozanimod were also more likely to have endoscopic improvement .
They found that the patients who had received a TNF inhibitor were also more likely to experience a clinical response with ozanimod than with adalimumab .
In both the induction and the maintenance phases, the other differences in efficacy between ozanimod and adalimumab did not reach statistical significance.
As for safety, in the induction phases of the trials, the researchers found that 11.3% of patients who received ozanimod had infections, compared to 20.2% of those taking adalimumab, which was statistically significant . Other differences in safety were not statistically significant.
For more news, follow Medscape on , , , and YouTube.
Don’t Miss: Ulcerative Proctitis Vs Ulcerative Colitis
Pk Of Ozanimod With Gemfibrozil Itraconazole Or Rifampin
This phase 1, randomized, open-label study focused on assessing the single-dose pharmacokinetics of ozanimod and its metabolites as well as to assess the effects of gemfibrozil, itraconazole, and rifampin on the single-dose PK of ozanimod. A total of 40 patients were randomized to receive either a single oral dose of ozanimod, oral doses of gemfibrozil + a single dose of ozanimod, oral doses of itraconazole + a single dose of ozanimod, or oral doses of rifampin + a single dose of ozanimod. In the single dose of ozanimod alone group, there were dose-proportional increases in Cmax and AUC for both the parent drug, ozanimod as well as its metabolites CC112273 and CC1084037. Itraconazole, a strong inhibitor of CYP3A and P-glycoprotein increased ozanimod AUC by 13%, while rifampin, a strong inducer of CYP3A and P-gp, reduced the AUC of ozanimod by 24%. This implies that there is a CYP3A4 and P-gp involvement in the metabolism of ozanimod. Gemfibrozil, a strong inhibitor of the CYP450 system, increased the AUC for the metabolites of ozanimod, CC112273 and CC1084037 by 47% and 69%, respectively. The metabolites of ozanimod were found to have similar single-dose PK properties, with CYP2C8 being the main enzyme in the metabolism of these metabolites, and CYP3A4 and P-gp being enzymes for the metabolism of ozanimod .
L.R. Fitzpatrick, T. Woldemariam, in, 2017
Pharmacokinetic Properties Of Ozanimod
Ozanimod and its major active metabolites CC112273 and CC1084037 demonstrate dose-proportional pharmacokinetics over an ozanimod dose range of 0.460.92 mg . In vitro data suggest that CC112273 is produced through monoamine oxidase B and metabolized via cytochrome P450 2C8 and oxido-reductases, and CC1084037 formed from CC112273 through direct reversible metabolism , with this interconversion favouring CC112273 . Other metabolic pathways for ozanimod include those of aldehyde dehydrogenase and alcohol dehydrogenase , CYP3A4 and 1A1, and gut microflora to form several other minor active metabolites , which have similar activity and selectivity for S1PR1 and S1PR5 to the parent drug and undergo further metabolism .
With multiple dosing, 94% of circulating total active substances consist of non-metabolized ozanimod , CC112273 and CC1084037 and the areas under the concentration-time curves of CC112273 and CC1084037 are 13-fold and 2.5-fold greater than that of ozanimod . Ozanimod, CC112273 and CC1084037 were bound 98.2%, 99.8%, and 99.3% to human plasma protein, respectively . The times to reach steady state for ozanimod and CC112273 in healthy individuals are 102 h and 45 days, respectively , and the time to reach maximum plasma concentration is 68 h for ozanimod and 10 h for CC112273 . Food intake in healthy individuals did not affect the exposure of ozanimod or its active metabolites .
Recommended Reading: Liver Disease Associated With Ulcerative Colitis
Ep: 14key Takeaways For Ulcerative Colitis Management
Miguel Regueiro, MD: In this section, were going to focus on S1P receptor modulators and other emerging treatments for ulcerative colitis . Ellen, were going to focus on ozanimod now, can you review some of the data from the phase 3 True North study with ozanimod? Give us the overview and any other information you want to add about ozanimod, and then Dave Ill move to you, and then Marla as well. Ellen, why dont you kick us off?
Miguel Regueiro, MD: Nice overview on ozanimod, this new mechanism of action, first in its class showing that there is benefit in the moderate to severe UC study. Nice job outlining the overall data related to True North and some of the more recent data on rapidity of onset with ozanimod, and now long-term extension. Im going to ask all 3 of you this, but Ill start with Dave and then Marla, can you tell me your experience with ozanimod? And Dave, if you want to add anything to what Ellen said about the trials, but I want to get into your experience in your clinics with ozanimod.
Miguel Regueiro, MD: Sometimes beer and wine too, patients ask. And I agree, to put that to rest, the amount of tyramine you would need to have is nearly impossible. I guess nothings impossible in eating. To Daves point about SSRIs, SNRIs, and showing safety, its the same thing with tyramine, its essentially a nonissue. Ellen, Im going to ask you the same question, how are you using ozanimod in your practice?
Transcript Edited for Clarity
The Efficacy And Safety Of Guselkumab Induction Therapy In Patients With Moderately To Severely Active Ulcerative Colitis: Phase 2b Quasar Study Results Through Week 12

The randomized, double-blind phase 2b QUASAR Induction Study 1 evaluated the safety and efficacy of 12 weeks of guselkumab in patients with moderately to severely active ulcerative colitis.1 The trial enrolled patients previously treated with conventional or advanced therapy that was intolerable or inadequate. Their Mayo rectal bleeding score was 1 or higher at baseline and their Mayo endoscopy subscore was at least 2, based on central review. The patients were randomly assigned to receive placebo, guselkumab at 200 mg every 4 weeks, or guselkumab at 400 mg every 4 weeks. The primary endpoint was the clinical response at week 12.
Among the entire study population of 313 patients, the median age was 41.6±14.40 years, and 59.1% were male. The mean duration of ulcerative colitis was 7.55±6.79 years. The mean Mayo score was 9.2±1.32, and the mean modified Mayo score was 7.0±1.0. Seventy percent of patients had a modified Mayo score of 7, 8, or 9, and 70% of patients had an endoscopy subscore of 3, indicating severe disease. Medications in use at baseline included oral aminosalicylates , oral corticosteroids , and immunosuppressants , and 23.3% of patients were intolerant to 2 or more classes of advanced therapy.
Reference
1. Dignass A, Rubin DT, Bressler B, et al. The efficacy and safety of guselkumab induction therapy in patients with moderately to severely active ulcerative colitis: phase 2b QUASAR study results through week 12 . J Crohns Colitis. 2022 16.
Don’t Miss: How To Heal Mouth Ulcers Fast
A Study To Evaluate Efficacy And Long
| The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government.Know the risks and potential benefits of clinical studies and talk to your health care provider before participating. Read our disclaimer for details. |
| Recruitment Status : Not yet recruitingFirst Posted : December 9, 2022Last Update Posted : December 9, 2022 |
| Study Type : | |
| Triple | |
| Primary Purpose: | Treatment |
| Official Title: | A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study of Oral Ozanimod to Evaluate Efficacy and Long-term Safety in Chinese Participants With Moderately to Severely Active Ulcerative Colitis |
| Estimated Study Start Date : |
| Specified dose on specified daysOther Name: BMS-986374 | |
| Placebo Comparator: Arm B: Placebo | Drug: PlaceboSpecified dose on specified days |