Abbvies Rinvoq Shows Promise As Maintenance Drug For Ulcerative Colitis
AbbVies Rinvoq hit the mark as a maintenance drug for ulcerative colitis in a Phase III study. The company said more patients treated with Rinvoq in the 52-week study achieved clinical remission compared to placebo.
Additionally, all secondary endpoints in the study were met, including the achievement of histologic-endoscopic mucosal improvement and corticosteroid-free clinical remission at one year, AbbVie said.
AbbVie noted that 49% of patients treated with 15 mg of Rinvoq and 62% of patients treated with 30 mg of the medication achieved endoscopic improvement at 52 weeks compared to 14% of patients in the placebo group. In addition, 35% of patients on 15 mg Rinvoq and 49% of patients on the 30 mg dose achieved HEMI compared to 12% of patients in the placebo group.
In the Phase III study, adult patients with moderate to severe ulcerative colitis who saw a clinical response to Rinvoq treatment following an eight-week study period of once-daily induction of 45 mg of the medication, were re-randomized to receive with 15mg or 30 mg of Rinvoq or placebo.
The dosing at these levels continued for one year. Full results from the Phase III maintenance study will be presented at a future medical meeting and submitted for publication in a peer-reviewed journal.
This is promising news for the IBD community, Panaccione said in a statement.
Featured Jobs on BioSpace
Medication Options For Ulcerative Colitis
Medication is the first line of treatment for ulcerative colitis. Your doctors recommendation for which medication will work best for you is based on the severity of your disease, your overall health, and other individual factors.
There are six major classes of medication used to treat ulcerative colitis.
The Potential Downsides Of Biologics
While biologics may be a promising option for treating your ulcerative colitis, not every drug is suited to every patient.
We will work with patients for a while to tailor a treatment plan that is right for them, says Dr. Raffals. That may mean trying different biologics and small-molecule drugs, and, of course, taking into account what the patients insurance will cover.
A persons lifestyle, demographic factors, and the severity of the disease are all considerations in the choice of whether or not to use biologics, and which of them is the best option.
Some potential downsides of taking biologics include:
If youre considering biologics for treatment, speak to your doctor to find out whats best for you. As Tsynman says, At the heart of the decision is the relationship between the patient and the physician and specifically exploring what works best for each individual.
Additional reporting by Jordan M. Davidson.
Read Also: How To Treat A Diabetic Foot Ulcer On The Sole
The Future Of Biosimilars
As of December 2019, a total of 26 biosimilars have been approved in the United States. But that number could grow quickly, according to Anita Afzali, MD, a gastroenterologist and the medical director of The Ohio State University Inflammatory Bowel Disease Center in Columbus.
There are more than 650 biosimilars under development, Dr. Afzali notes, adding that most are in the early stages of development. Whats to come in the United States will certainly be a hot topic of discussion.
So far in the United States, biosimilars for IBD have been approved for all the same uses as their originator drugs but have not been ruled interchangeable with those drugs. In practice, this means that pharmacists cant substitute a biosimilar for a biologic, as they could with a generic for a brand-name drug.
Afzali worries that this may become a distinction without a difference. Its easy to imagine, she says, that an insurance company or hospital system could simply cover only the biosimilar equivalent of an original biologic. In this imagined scenario, she says, the state or the insurance or the pharmacist is saying, You have to try the biosimilar, even when a person has already experienced side effects from the biologic that might become worse with the biosimilar.
Tofacitinib: New Drug Approved For The Treatment Of Patients With Ulcerative Colitis
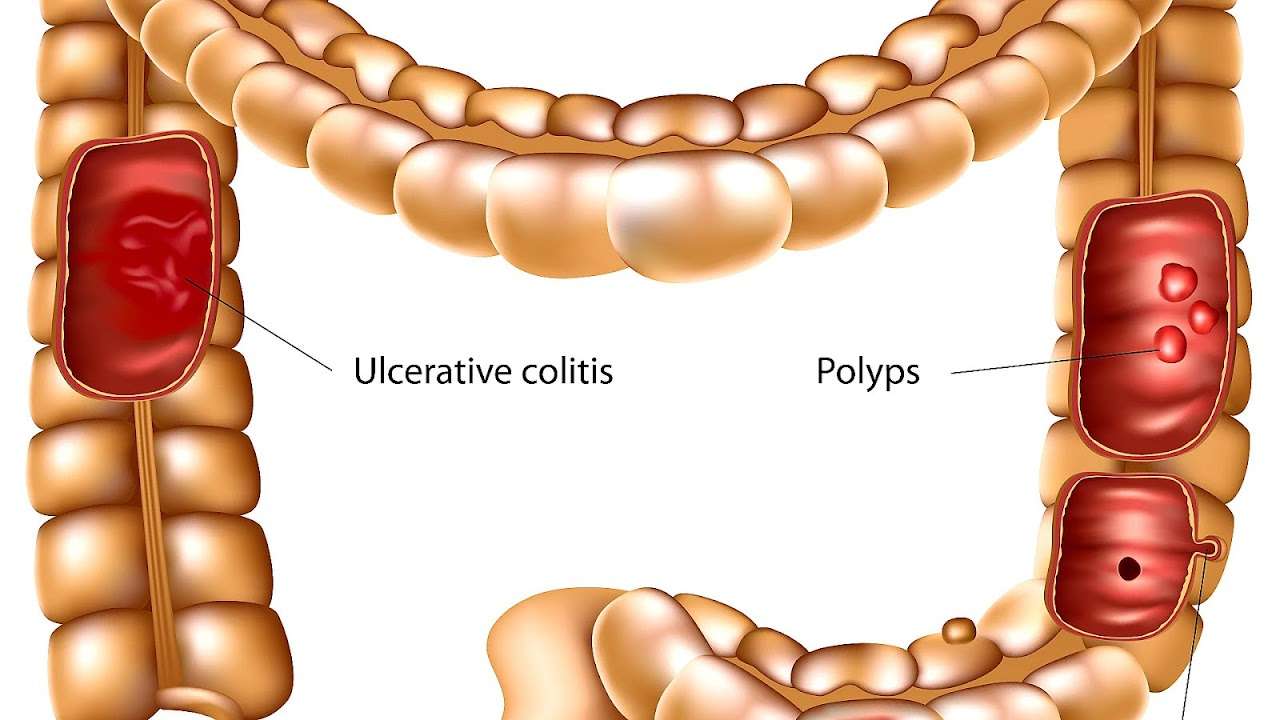
Ulcerative colitis is a chronic inflammatory disease that affects the colonic mucosa. There is no curative treatment. Instead, patients must take medication to control the inflammation over the course of their lives. There are some patients whose condition cannot be sufficiently controlled by the drugs currently available. For them, the new treatment with tofacitinib could be an alternative.
In 2017, the European Medicines Agency approved tofacitinib for the treatment of ulcerative colitis in adult patients who presented an intolerance, insufficient response or loss of response to conventional treatment or biological medical products , which are also commonly administered to treat ulcerative colitis.
Tofacitinibs mechanism of action is different to all other currently approved molecules: it inhibits a route of inflammation known as JAK . Inhibiting this route of action represents a new approach in the treatment of ulcerative colitis.
This treatment has many advantages in terms of its administration: it is administered orally, and this encourages patients to accept and follow the treatment. Furthermore, it has a fast onset of action, the effect of the drug only lasts for a few hours in the body, there is no risk of producing antibodies against it and it is used in monotherapy, in other words, there is no need to combine it with other drugs.
- Keep reading about:
Recommended Reading: Is Nexium Good For Ulcers
Fda Approves New Drug For Ulcerative Colitis
- Federal regulators have approved the new drug Zeposia for treating moderate to severe ulcerative colitis in adults.
- The medication is the latest in a line of drugs used to treat symptoms of this particular type of inflammatory bowel disease .
- Clinical trials are under way to test Zeposias effectiveness in treating Crohns disease.
If youre living with ulcerative colitis, you may have a new treatment option.
On May 27, Bristol Myers Squibb officials announced that the Food and Drug Administration approved Zeposia for treatment of moderate to severe active ulcerative colitis in adults.
Ulcerative colitis is a chronic inflammatory bowel disease . Symptoms such as abdominal pain, diarrhea, and malnutrition can affect a persons quality of life.
Currently, the only potential cure for ulcerative colitis is surgery to remove the colon. But there are several types of medications to help manage the disease.
An estimated 3 million adults in the United States have been diagnosed with ulcerative colitis or Crohns disease, another type of IBD.
The FDA approved Zeposia in 2020 as a disease-modifying therapy for adults with relapsing forms of multiple sclerosis. And phase 3 trials are under way to evaluate the safety and efficacy of Zeposia for treating Crohns disease.
Fda Approves Ozanimod For Ulcerative Colitis
Megan Brooks
May 28, 2021
The US Food and Drug Administration has approved ozanimod for adults with moderately to severely active ulcerative colitis , the company has announced.
Ozanimod , an oral medication taken once daily, is the first sphingosine-1-phosphate receptor modulator approved for UC.
In March 2020, the FDA approved ozanimod for adults with relapsing forms of multiple sclerosis, as reported by Medscape Medical News.
The approval of ozanimod for UC is based on data from True North, a phase 3 placebo-controlled trial that evaluated the drug as induction therapy over 10 weeks in 645 adults with moderately to severely active UC, followed by maintenance therapy over 42 weeks.
All participants in the trial had failed to respond adequately to or were intolerant of oral aminosalicylates, corticosteroids, immunomodulators, or a biologic 30% had previously experienced treatment failure or were intolerant of tumor necrosis factor blockers. Prior to and during induction, patients were treated with oral aminosalicylates and/or corticosteroids.
During induction at week 10, the trial met its primary endpoint significantly more patients who took ozanimod achieved clinical remission than patients who took placebo .
Ozanimod was also superior to placebo on the secondary endpoints of clinical response , endoscopic improvement , and endoscopic-histologic mucosal improvement .
Complete prescribing information and medication guide are available online.
Send news tips to .
Also Check: Bleeding Ulcer Treatment At Home
Stanford Scientists Link Ulcerative Colitis To Missing Gut Microbes
Bacteria normally inhabiting healthy peoples intestines and the anti-inflammatory metabolites these bacteria produce are depleted in ulcerative colitis patients, a Stanford study shows.
Aida Habtezion is the senior author of a study that describes how people with ulcerative colitis have insufficient amounts of a metabolite produced by a family of gut-dwelling bacteria.Steve Castillo
About 1 million people in the United States have ulcerative colitis, a serious disease of the colon that has no cure and whose cause is obscure. Now, a study by Stanford University School of Medicine investigators has tied the condition to a missing microbe.
The microbe makes metabolites that help keep the gut healthy.
This study helps us to better understand the disease, said Aida Habtezion, MD, associate professor of gastroenterology and hepatology. We hope it also leads to our being able to treat it with a naturally produced metabolite thats already present in high amounts in a healthy gut.
When the researchers compared two groups of patients one group with ulcerative colitis, the other group with a rare noninflammatory condition who had undergone an identical corrective surgical procedure, they discovered that a particular family of bacteria was depleted in patients with ulcerative colitis. These patients also were deficient in a set of anti-inflammatory substances that the bacteria make, the scientists report.
The Zeposia 360 Support Program Can Help You Every Step Of The Way
Have questions about ZEPOSIA or starting treatment? With ZEPOSIA 360 Support, a UC Nurse Navigator is there to make sure you have all the answers you need when it comes to ZEPOSIA. A dedicated UC Nurse Navigator can also help with insurance benefits, financial support, and so much more, including determining if you’re eligible for the ZEPOSIA $0 co-pay offer.#
#Depending on insurance coverage, patients may be eligible to receive a prescription benefit offer for out-of-pocket drug costs and pay as little as $0 per prescription. Maximum savings limit applies patient out-of-pocket expenses may vary. This program is not health insurance. Offer not valid for patients enrolled in Medicare, Medicaid, or other federal or state health care programs. Please for Program Terms, Conditions, and Eligibility Criteria.
Wondering if ZEPOSIA is right for you? Here are 3 things you can do to get started:
- Connect with your healthcare provider. Here are some questions to get the conversation started
- Visit ZEPOSIA.com to learn more about ZEPOSIA and the ZEPOSIA 360 Support program
- Connect with a UC Nurse Navigator by calling 1-833-ZEPOSIA , Monday to Friday, 8 AM – 8 PM ET
Read Also: How Long Does Prednisolone Take To Work For Ulcerative Colitis
New Drug Effective In Ulcerative Colitis
Stephen Hanauer, MD, the Clifford Joseph Barborka Professor and a co-author of the study published in the New England Journal of Medicine.
A new drug improved management of ulcerative colitis, according to a study in the New England Journal of Medicine.
Patients treated with ozanimod, a sphongosine 1 phosphate receptor modulator that prevents inflammation, experienced a higher rate of remission compared to patients who received a placebo.
This is a safe and effective oral option for patients with moderate-severe disease, said Stephen Hanauer, MD, the Clifford Joseph Barborka Professor of Medicine in the Division of Gastroenterology and Hepatology and a co-author of the study.
Ulcerative colitis is an inflammatory bowel disease, characterized by inflammation and sores in the innermost lining of the colon and rectum. There is no cure and while current pharmacological therapies are effective in mild or moderate disease, options for severe disease are lacking, according to Hanauer.
There are several biologic and small molecules now available for moderate-severe ulcerative colitis, but none as effective as we would like, Hanauer said.
Ozanimod is a sphingosine-1-phosphate receptor modulator, causing S1P1 receptors to internalize in lymphocytes, preventing those immune cells from mobilizing to inflammatory sites.
It traps lymphocytes in lymph nodes so they are unable to get into the colon to cause tissue damage, Hanauer said.
Analyzing Genes And Drugs Effects On Them
Khatri and his team began their research by analyzing publicly available genomic data from hundreds of patients with ulcerative colitis who had undergone a colon biopsy, a somewhat common practice that helps doctors diagnose the disease and its severity. Specifically, Khatri and his team were looking for certain genomic signatures, or patterns of gene activity, that seemed to persist in most patients with the condition.
We looked at national and international data, and we found a disease signature that was robust across all the datasets irrespective of whether the patient was experiencing a flare in disease, Khatri said.
From there, it was matter of identifying how certain drugs affected the gene activity associated with ulcerative colitis. Khatri turned to data from previously conducted lab studies in cells that showed how certain drugs changed the activity of genes. The idea was to find the drugs that seemed to reverse the gene signature associated with ulcerative colitis. For instance, if patients with ulcerative colitis had a dip in the activity of gene A and B, the team looked for drugs that increased activity in those genes. They looked only at drugs that had been approved by the Food and Drug Administration so that, if they found a drug that worked, it could be rolled out to patients sooner.
Also Check: Chances To Win Social Security Disability With Ulcerative Colitis
What You Need To Know
Zeposia is an oral medication taken once a day. The dose is 0.92 milligrams.
Dr. Rudolph Bedford, a gastroenterologist at Providence Saint Johns Health Center in Santa Monica, told Healthline that Zeposia is a potential game changer for people with ulcerative colitis who dont respond to traditional therapies.
Traditional therapies include aminosalicylates along with corticosteroids and immunomodulators. Theyre all oral therapies, but quite often theres no response or an ineffective response, so we move on to biologics, he said.
Zeposia is a sphingosine 1-phosphate receptor modulator.
In ulcerative colitis and Crohns disease, T-cells attack the mucosal lining of the colon. This modulator regulates how that process occurs, Bedford explained. By essentially eliminating T-cells from moving into the lining of the colon, it prevents an inflammatory response with bleeding, diarrhea, and everything else that goes along with ulcerative colitis.
Weve been looking forward to oral medications coming out that are completely different from what were used to, he said. Im not sure its a first-line therapy at this point, but there will likely be more studies looking at this in naïve patients. I suspect that eventually the medical community will start to embrace it.
However, Zeposia isnt for everyone with ulcerative colitis.
The drug was also contraindicated in those who have:
- Mobitz type II second-degree or third degree atrioventricular block
- sick sinus syndrome
A New Indication Of Success: Fda Approves Ozanimod For Ulcerative Colitis

The novel drug created at Scripps Research has achieved a second FDA approval, this time for ulcerative colitis, as clinical trials continue for Crohns disease.
May 27, 2021
LA JOLLA, CAOzanimod, the drug invented at Scripps Research that won FDA approval last year for relapsing forms of multiple sclerosis, has been approved in the United States for a second high-need medical condition, ulcerative colitis.
The once-daily oral drug, sold by Bristol Myers Squibb under the name Zeposia, can now be prescribed to treat adults with moderate to severe forms of the inflammatory bowel disease. Notably, its the first drug in a novel class of immune-modulating compounds to be approved for ulcerative colitis, which affects about 1 million people in the United States.
For patients with ulcerative colitis, this oral drug offers a better and more convenient option to control disease progression and improve quality of life, says Hugh Rosen, MD, PhD, who invented ozanimod along with fellow Scripps Research professor Edward Roberts, PhD, and their laboratory colleagues. The hope is that this will lead to fewer dangerous complications or serious infections than current treatment options, providing a steadier path for newly diagnosed patients as well as those failing other treatments.
Additional molecules developed by Rosen and Roberts at Scripps Research are currently in phase 2 clinical trials for major depressive disease and anxiety, and phase 1 studies for treatment of autism.
Read Also: How Do You Heal An Ulcer
The Positive Side Of Biosimilars: More Ibd Treatment Choices
No one doubts that biosimilars are here to stay and that theyll take over a growing share of the biologic drug market in coming years.
Afzali hopes the influx of new drugs will prompt more research on their effects in people with IBD. We still need a lot more human and research testing, she says, to really evaluate the safety and efficacy of biosimilars for individual diseases.
Gerich is cautiously optimistic about the potential benefits of biosimilars. Hopefully, safety wont be a concern, he says, and it will be interesting to see what the financial outcomes are.
As Afzali notes, as long as choices arent taken away from doctors and patients, the availability of biosimilars can only be a good thing. I think having more treatment options in our medicine cabinet is always appealing, she says.
Additional reporting by Quinn Phillips
Are Biosimilars Effective At Treating Ibd
As noted above, biosimilars arent exact copies of the drugs theyre modeled after. When a compound has extremely large molecules that are created through a biological process, its impossible to re-create that compound exactly. If the FDA adopted such a standard for the approval of biosimilars, it would never be met.
So instead of showing that theyre identical to the original drug on a molecular level, which is required for generic drugs, manufacturers of biosimilars must prove that theyre similar enough to the originals to have no clinically meaningful difference. This is done through clinical trials for safety and effectiveness, which account for a large share of the cost of bringing a biosimilar to market.
But according to the FDAs rules, biosimilars are required to be tested for only one indication disease or health condition that the originator drug is approved for. If the drug is shown to be safe and effective for this use, the FDA extrapolates that its safe and effective for all approved uses of the originator drug.
For example, if a biologic drug is approved for treating rheumatoid arthritis, plaque psoriasis, UC, and Crohns disease, and a biosimilar version is shown to be safe and effective for rheumatoid arthritis, then the FDA will approve the biosimilar for all the other conditions as well, without testing the drug in those patient populations.
Don’t Miss: Are Ulcerative Colitis And Ibs The Same