What You Need To Know
Zeposia is an oral medication taken once a day. The dose is 0.92 milligrams.
Dr. Rudolph Bedford, a gastroenterologist at Providence Saint Johns Health Center in Santa Monica, told Healthline that Zeposia is a potential game changer for people with ulcerative colitis who dont respond to traditional therapies.
Traditional therapies include aminosalicylates along with corticosteroids and immunomodulators. Theyre all oral therapies, but quite often theres no response or an ineffective response, so we move on to biologics, he said.
Zeposia is a sphingosine 1-phosphate receptor modulator.
In ulcerative colitis and Crohns disease, T-cells attack the mucosal lining of the colon. This modulator regulates how that process occurs, Bedford explained. By essentially eliminating T-cells from moving into the lining of the colon, it prevents an inflammatory response with bleeding, diarrhea, and everything else that goes along with ulcerative colitis.
Weve been looking forward to oral medications coming out that are completely different from what were used to, he said. Im not sure its a first-line therapy at this point, but there will likely be more studies looking at this in naïve patients. I suspect that eventually the medical community will start to embrace it.
However, Zeposia isnt for everyone with ulcerative colitis.
The drug was also contraindicated in those who have:
- Mobitz type II second-degree or third degree atrioventricular block
- sick sinus syndrome
Bristol Myers Squibb Presents Interim Results From Long
The percentage of patients achieving clinical remission, clinical response, endoscopic improvement and corticosteroid-free remission was maintained through Week 142
Zeposia is the first and only oral sphingosine 1-phosphate receptor modulator approved to treat patients with ulcerative colitis
PRINCETON, N.J.â-Bristol Myers Squibb today announced interim results from the True North open-label extension study evaluating the long-term efficacy and safety profile of Zeposia in patients with moderately to severely active ulcerative colitis . Findings show that the percentage of patients achieving clinical remission, clinical response, endoscopic improvement and corticosteroid-free remission was maintained through Week 142. No new safety signals emerged in the study. These data will be presented at the 17th Congress of the European Crohnâs and Colitis Organisation , taking place February 16-19, 2022.
Additional Bristol Myers Squibb-sponsored abstracts presented at the ECCO 2022 Congress can be accessed online here.
Visit this page on BMS.com for more information on Bristol Myers Squibbs scientific approach and resources on gastrointestinal immune-mediated diseases.
About True North
Bristol Myers Squibb thanks the patients and investigators involved in the True North clinical trial.
About Ulcerative Colitis
ZEPOSIA is indicated for the treatment of:
IMPORTANT SAFETY INFORMATION
Read Also: Foam Dressings For Leg Ulcers
Sphingosine 1 Phosphate Receptor Modulator
Shingosine-1 phosphate signaling on central memory T-cells facilitate their exit from lymph nodes. Internalization of the S1P receptor prevents lymphocytes from responding to S1P and are retained in the lymph node, thus inhibiting their recruitment to inflamed tissue. Protective immunity is generally preserved as effector memory T-cells do not circulate through the lymph nodes. Ozanimod is an S1P modulator which downregulates S1P receptor subtypes 1 and 5. In a phase 2 RCT in patients with moderately to severely active UC, 1mg of ozanimod showed significantly greater clinical response and remission rates compared with placebo . In this study, endoscopic remission rates at week 8 were also significantly greater in the treatment groups. The adverse event profile was similar to placebo. The 4-year open label extension study showed durable efficacy with no new safety markers in UC patients. The phase 3 TRUE NORTH study in patients with moderately to severely active UC showed that ozanimod results in significant benefits in clinical, endoscopic, histologic and mucosal healing endpoints at week 52 compared with placebo. Currently, phase 3 trials in CD and UC patients are underway.
Don’t Miss: What Foods To Eat When You Have A Stomach Ulcer
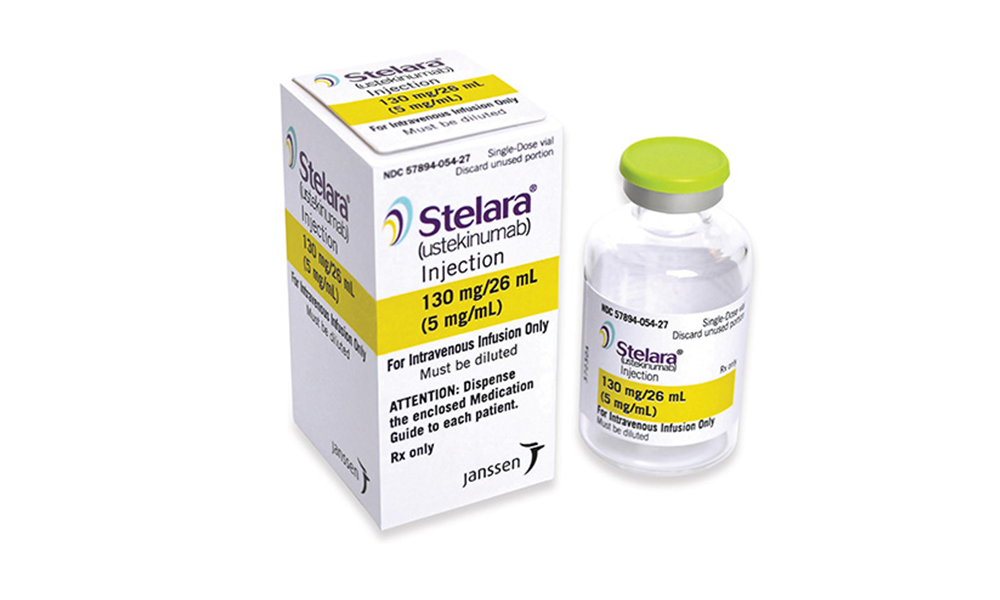
How Is Stelara Given
- In ulcerative colitis in adults, Stelara is initially given as a one-time, weight-based intravenous infusion dose.
- It is followed by a maintenance injection dose of 90 mg given by a subcutaneous injection every 8 weeks.
In studies, most patients saw rapid relief from UC symptoms in 8 weeks, with 20% of patients reaching remission. At one year, 40% of patients were in remission. At 2 years, 70% of patients had no rectal bleeding and fewer daily bowel movements.
The most common side effects in ulcerative colitis studies include:
- Nasopharyngitis
Drugs That Target Inflammation
Most people with UC take prescription drugs called aminosalicylates that tame inflammation in the gut. These include balsalazide , mesalamine , olsalazine , and sulfasalazine . Which one you take, and whether it is taken by mouth or as an enema or suppository, depend on the area of your colon that’s affected. As long as you avoid your triggers, these may be enough if your disease is mild to moderate.
You may need something else if your condition is more severe or if those standard treatments stop working. Your doctor may consider other medicines. Some people may also need surgery.
Recommended Reading: Can Ulcers Cause Black Stool
The Positive Side Of Biosimilars: More Ibd Treatment Choices
No one doubts that biosimilars are here to stay and that theyll take over a growing share of the biologic drug market in coming years.
Afzali hopes the influx of new drugs will prompt more research on their effects in people with IBD. We still need a lot more human and research testing, she says, to really evaluate the safety and efficacy of biosimilars for individual diseases.
Gerich is cautiously optimistic about the potential benefits of biosimilars. Hopefully, safety wont be a concern, he says, and it will be interesting to see what the financial outcomes are.
As Afzali notes, as long as choices arent taken away from doctors and patients, the availability of biosimilars can only be a good thing. I think having more treatment options in our medicine cabinet is always appealing, she says.
Additional reporting by Quinn Phillips
Who Is A Candidate For Jak Inhibitors
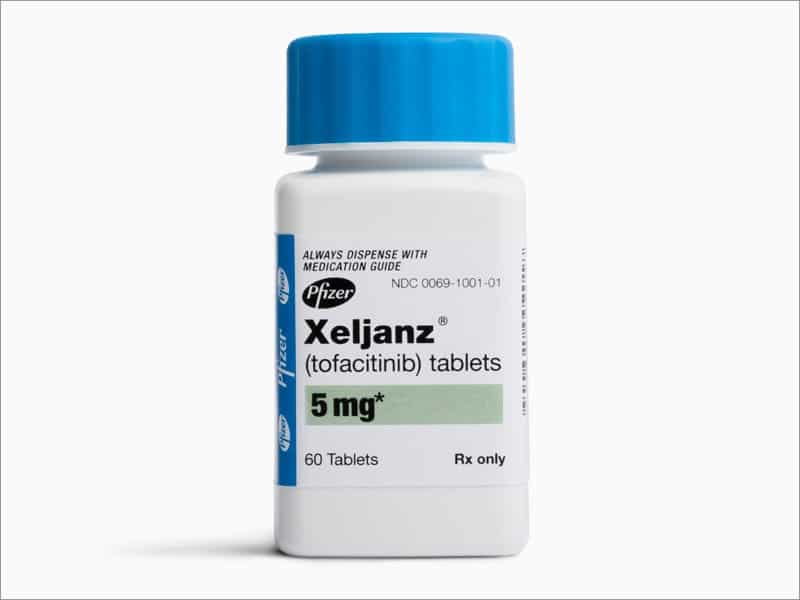
The FDA has approved Xeljanz and Rinvoq to treat moderate to severe UC.
However, a doctor will likely try another type of medication before prescribing a JAK inhibitor for this condition.
In its 2020 guidelines, the American Gastroenterological Association recommended infliximab or vedolizumab as the first-line treatment for moderate to severe UC. Both of these medications are biologics, which is a different class of medication than JAK inhibitors.
If a person does not have an adequate or lasting response to treatment with Remicade, Entyvio, or other biologics, the doctor may prescribe another medication, such as Xeljanz or Rinvoq.
A doctor may also prescribe Xeljanz or Rinvoq to someone who has RA or psoriatic arthritis in addition to UC. The FDA has approved these medications to treat all three conditions.
JAK inhibitors may not be a suitable treatment option for all people with moderate to severe UC, though.
I would not recommend it for patients who are pregnant or breastfeeding, said Dr. Mone. I would use caution in patients with a history of blood clots or heart problems. I would also avoid in patients with active malignancy.
Before taking JAK inhibitors, people should ensure that the prescribing doctor is aware of any other medical conditions they have, particularly:
- active infection
Don’t Miss: What To Take For Stomach Ulcer Pain
Tofacitinib: New Drug Approved For The Treatment Of Patients With Ulcerative Colitis
Ulcerative colitis is a chronic inflammatory disease that affects the colonic mucosa. There is no curative treatment. Instead, patients must take medication to control the inflammation over the course of their lives. There are some patients whose condition cannot be sufficiently controlled by the drugs currently available. For them, the new treatment with tofacitinib could be an alternative.
In 2017, the European Medicines Agency approved tofacitinib for the treatment of ulcerative colitis in adult patients who presented an intolerance, insufficient response or loss of response to conventional treatment or biological medical products , which are also commonly administered to treat ulcerative colitis.
Tofacitinibs mechanism of action is different to all other currently approved molecules: it inhibits a route of inflammation known as JAK . Inhibiting this route of action represents a new approach in the treatment of ulcerative colitis.
This treatment has many advantages in terms of its administration: it is administered orally, and this encourages patients to accept and follow the treatment. Furthermore, it has a fast onset of action, the effect of the drug only lasts for a few hours in the body, there is no risk of producing antibodies against it and it is used in monotherapy, in other words, there is no need to combine it with other drugs.
- Keep reading about:
Ulcerative Colitis Treatment: Medication
The most common medication option is anti-inflammatory drugs. These can be used orally or topically to reduce inflammation of the colon and rectum.
Treating ulcerative colitis is a highly individualized process. At Johns Hopkins, we tailor your treatment to your specific needs and alter the medication as necessary. Your specific medication regimen will depend largely on the severity of your condition.
Other medications include:
-
Immunosuppressive medications: These drugs slow your immune system to stop the immune response that is causing the colon and rectum to swell.
-
Biologics: Like immunosuppressive medications, biologics target the immune system, but biologics act on specific immune system proteins that encourage inflammation.
You May Like: Is Ulcerative Colitis Worse Than Crohn Disease
How Do People Take Jak Inhibitors
JAK inhibitors are oral medications that people swallow.
The standard dosage of Xeljanz is 10 mg twice per day for at least 8 weeks to induce remission in UC. However, in some cases, a persons doctor may prescribe a lower dosage.
Once a person is in remission, the doctor will reduce their prescribed dosage of Xeljanz to the smallest amount necessary to maintain remission.
The recommended dosage for adults taking Rinvoq is 45 mg once per day for 8 weeks to induce remission. The recommended dosage to maintain remission is 15 mg once a day. People with severe or extensive UC may need a different maintenance dosage.
A person should work with their doctor to determine the JAK inhibitor dosage that is right for them.
Sometimes, a doctor may prescribe a JAK inhibitor in combination with other medication to manage UC.
What Is Ulcerative Colitis
Ulcerative colitis is one of the more common IBDs, but microscopic colitis and Crohns disease are also fairly common examples in the category . They all share some common symptoms, but they each have specific characteristics that set them apart.
Ulcerative colitis is focused on and limited to the large intestinealso known as the colon. The key characteristics that set it apart from Crohns disease is the irritation, swelling, and sores on the inner lining of the colon. Crohns disease, by comparison, can affect other parts of the gastrointestinal tract such as the small intestine, and it can be limited to certain areas. Ulcerative colitis is a more broad inflammation of the inner lining of the entire colon.
One of the other main characteristics of ulcerative colitis is that it is chronic. When doctors refer to a condition as chronic, they are referring to something that is long-lasting or persistent. For many sufferers of ulcerative colitis , the symptoms tend to recur, and there is currently no known cure. The best hope is a long term remission.
Don’t Miss: How To Heal Stage 4 Pressure Ulcer
A New Indication Of Success: Fda Approves Ozanimod For Ulcerative Colitis
The novel drug created at Scripps Research has achieved a second FDA approval, this time for ulcerative colitis, as clinical trials continue for Crohns disease.
May 27, 2021
LA JOLLA, CAOzanimod, the drug invented at Scripps Research that won FDA approval last year for relapsing forms of multiple sclerosis, has been approved in the United States for a second high-need medical condition, ulcerative colitis.
The once-daily oral drug, sold by Bristol Myers Squibb under the name Zeposia, can now be prescribed to treat adults with moderate to severe forms of the inflammatory bowel disease. Notably, its the first drug in a novel class of immune-modulating compounds to be approved for ulcerative colitis, which affects about 1 million people in the United States.
For patients with ulcerative colitis, this oral drug offers a better and more convenient option to control disease progression and improve quality of life, says Hugh Rosen, MD, PhD, who invented ozanimod along with fellow Scripps Research professor Edward Roberts, PhD, and their laboratory colleagues. The hope is that this will lead to fewer dangerous complications or serious infections than current treatment options, providing a steadier path for newly diagnosed patients as well as those failing other treatments.
Additional molecules developed by Rosen and Roberts at Scripps Research are currently in phase 2 clinical trials for major depressive disease and anxiety, and phase 1 studies for treatment of autism.
What Are The Potential Risks Of Taking Jak Inhibitors
Research has shown that JAK inhibitors are generally safe for people with UC, but they do carry the risk of side effects.
If a person thinks that they might be experiencing side effects from a JAK inhibitor or another medication, they should speak with the prescribing doctor.
Doctors can order regular lab tests to check for signs of side effects from JAK inhibitors.
You May Like: My Ulcerative Colitis Is Getting Worse
Rinvoq Receives Fda Approval For The Treatment Of Adults With Moderately To Severely Active Ulcerative Colitis
NORTH CHICAGO, Ill., March 16, 2022 /PRNewswire/ — AbbVie today announced that the U.S. Food and Drug Administration has approved Rinvoq® for the treatment of adults with moderately to severely active ulcerative colitis who have had an inadequate response or intolerance to one or more tumor necrosis factor blockers. This FDA approval is the first indication for Rinvoq in gastroenterology and is supported by efficacy and safety data from three Phase 3 randomized, double-blind, placebo-controlled clinical studies.
“There remains an unmet need for patients with moderately to severely active UC, who suffer from debilitating symptoms that are often unpredictable and burdensome,” said Thomas Hudson, MD, senior vice president of research and development, chief scientific officer, AbbVie. “With the approval of Rinvoq as a new treatment option, AbbVie continues our leadership in advancing research that can help impact the lives of people living with ulcerative colitis.”
Clinical Response and Durable Remission1-4
Endoscopic Improvement and Mucosal Healing1-4
Rinvoq Safety Profile4
*Dr. Abreu is a consultant and advisor for AbbVie.
Rinvoq U.S. Use and Important Safety Information4
Rinvoq is a prescription medicine used:
The Founding Of Evinature
After the success of their trial, Salomon and Ben-Horin founded Evinature with an aim to promote safe, clinically validated natural remedies. The patient-focused platform provides integrative strategies, guided care from a collective of integrative and GI experts, research updates, and vital information to the community.
The Gold Standard in Evidence-based Natural Remedies
You May Like: What Can I Take For Ulcerative Colitis
Recommended Reading: Mouth Ulcer On Gum Causes
Mm Ulcerative Colitis Market Forecasts Epidemiology & Pipeline Analysis 2022
DUBLIN, Sept. 2, 2022 /PRNewswire/ — The “Ulcerative Colitis Market Forecast – Epidemiology & Pipeline Analysis 2022-2027” report has been added to ResearchAndMarkets.com’s offering.
The ulcerative colitis epidemiology provides insights into the historical and current patient pools and forecasted trends for eight major countries.
The ulcerative colitis segment covers the historical and forecasted epidemiology data in the US, EU5 , China, and Japan from 2021-2027. The Ulcerative colitis epidemiology data are studied through all possible divisions to give a better understanding of the disease scenario in 8MM.
This report provides a detailed analysis of the drugs available for the treatment of ulcerative colitis. The report covers a detailed drug description including drug name, sponsor name, route of administration, molecule type, mechanism of action of the drug, clinical studies, NDA approvals, and Ulcerative colitis collaborations, licensing, mergers and acquisition, designations, and other product-related details.
According to estimates, the diagnosed incident cases of inflammatory bowel disease were high in the United States, followed by China and the UK in 2020. The increasing prevalence of ulcerative colitis is driving the growth of the ulcerative colitis drug market. According to the estimates, one million people were affected by inflammatory bowel disease in the US in 2019.
GEOGRAPHY ANALYSIS
ULCERATIVE COLITIS THERAPEUTICS: CLINICAL TRIALS SCENARIO
Key Vendors
Fda Approves Boxed Warning About Increased Risk Of Blood Clots And Death With Higher Dose Of Arthritis And Ulcerative Colitis Medicine Tofacitinib
FDA Drug Safety Podcast
Welcome to the FDA Drug Safety Podcast for health care professionals from the Division of Drug Information. This is Lesley Navin Advanced Practice Nurse.
On July 26, 2019, FDA approved new warnings about an increased risk of blood clots and death with the 10 mg twice daily dose of tofacitinib , used in patients with ulcerative colitis. The approved use of tofacitinib for ulcerative colitis will also be limited to patients who are not treated effectively or who experience severe side effects with certain other medicines. We approved these changes, adding our most prominent Boxed Warning, after reviewing interim data from an ongoing safety clinical trial of tofacitinib in patients with rheumatoid arthritis that examined a lower and this higher dose of the medicine.
The 10 mg twice daily dose of tofacitinib is only approved for initial treatment of ulcerative colitis and for long-term use in limited situations. While the increased risks of blood clots and death were seen in patients taking this dose for RA, these risks may also apply to those taking tofacitinib for ulcerative colitis.
Tofacitinib was approved in 2012 to treat adult patients with RA who did not respond well to methotrexate. In 2017, we approved Tofacitinib to treat patients with psoriatic arthritis, who did not respond well to other medicines and in 2018, to treat ulcerative colitis.
And follow us on Twitter @FDA_Drug_Info for up to the minute important drug information.
Read Also: Best Ointment For Leg Ulcers