Receiving Remicade With Other Drugs
If you have RA, your doctor can only prescribe Remicade with methotrexate.
Flare-ups of inflammatory diseases may require taking corticosteroids.
People using Remicade with methotrexate or corticosteroids have a higher risk for developing a serious infection. This is because Remicade, methotrexate, and corticosteroids are all immunosuppressants. They reduce the activity of the immune system, decreasing the ability of your body to fight infections.
Other medications you may need to take with Remicade include:
- aminosalicylates, including mesalamine and sulfasalazine
Dont Miss: How Is Ulcerative Colitis Caused
Infusion Dosing Schedule In Adults And Children
Even if you will be taking infliximab by injection, youll have your first two doses by infusion in hospital. See the previous sections on infusions.
Youll usually have your first injection in hospital or by a trained nurse at home. Youll then be trained to inject it yourself. If you prefer, it may be possible for someone else, such as a family member, to be trained to give you the injections.
Infliximab comes ready to use in either a pre-filled syringe or a pre-filled injection pen. You may not see the needle in the injection pen, as its inside. The syringes or pens come in a pack. The pack contains an alcohol pad to clean your skin before injecting.
The Advantages Of Biologics For Ulcerative Colitis
Biologics can improve gut symptoms, bringing about and maintaining remission in people with moderate to severe ulcerative colitis. They can also reduce the need for hospitalization and surgery, says the British organization Crohns & Colitis UK.
One key advantage of biologic therapies over other types of treatment for ulcerative colitis is that their mechanisms of action are more precisely targeted to the factors responsible for the condition, notes the Crohns & Colitis Foundation.
Unlike corticosteroids, for example, which affect the whole body and may produce major side effects, biologic agents act more selectively. These therapies are targeted to particular proteins that have already been proven to be involved in ulcerative colitis.
Patients are often nervous about biologics, says Laura Raffals, MD, a gastroenterologist at the Mayo Clinic in Rochester, Minnesota. But theyre not as scared about taking a corticosteroid, and thats an important conversation to have because the data shows that patients on biologics are much safer and stay in remission longer. The serious side effects we see are from steroids or narcotics, not biologics.
Also Check: Ulcerative Colitis Surgery J Pouch
Accent I Study Design
ACCENT I is a 1-year, multicenter, randomized, double-blind trial of REMICADE® in 545 patients with moderately to severely active CD . All patients received an initial dose of REMICADE® 5 mg/kg IV. Patients were then randomized based on clinical response at Week 2 to 1 of 3 treatment groups through Week 541,6:
- The placebo maintenance group received placebo infusion at Weeks 2, 6, and every 8 weeks thereafter
- The 5 mg/kg IV maintenance group received REMICADE® 5 mg/kg IV at Weeks 2, 6, and every 8 weeks thereafter
- The REMICADE® 10 mg/kg IV maintenance group received REMICADE® 5 mg/kg IV at Weeks 2 and 6, followed by REMICADE® 10 mg/kg IV every 8 weeks thereafter
The coprimary endpoints of the trial were the proportion of patients responding at Week 2 who were in remission at Week 30 and time to loss of response through Week 54.1,6
Note: The recommended dose of REMICADE® is 5 mg/kg given as an IV induction regimen at 0, 2, and 6 weeks followed by a maintenance regimen of 5 mg/kg IV every 8 weeks thereafter for the treatment of adults with moderately to severely active Crohnâs disease.1
Read Also: How To Gain Weight With Ulcerative Colitis
Telling Other Health Professionals
Tell any doctor, dentist or health professional treating you that you are taking infliximab. Always carry the alert card that comes with the medicine while you are taking it and for up to six months after your last dose.
Its not safe to have live vaccines while taking infliximab. It can take up to six months after your last dose for infliximab to completely leave your body. However, its safe to have live vaccines 3 months after your last dose of infliximab. Ask your IBD team to make sure your vaccinations are up to date before you start infliximab, or if youre planning to travel. If youve recently had a live vaccine you may have to wait 4 weeks before starting infliximab.
In the UK, live vaccines include:
- BCG
- Rotavirus
- Flu nasal spray
Everyone with Crohns or Colitis taking a biologic medicine should have the yearly flu jab. This is not a live vaccine and is safe to have while taking infliximab.
If someone you live with is due to have a live vaccine, ask your IBD team if you need to take any precautions.
Infliximab does not affect fertility. If you dont want to get pregnant you should use contraception.
You must tell your babys healthcare team you were taking infliximab while pregnant. It is advised that if you take infliximab during your pregnancy your baby should avoid live vaccines until they are at least six months old. This includes the BCG for tuberculosis and the rotavirus vaccine. It should not affect the rest of your babys vaccination schedule.
Don’t Miss: What Does Venous Stasis Ulcer Look Like
Is Entyvio Used Long Term
Yes, Entyvio is meant to be used as a long-term treatment. How long youll use Entyvio for depends on whether the drug is working to manage the symptoms of your condition. If you and your doctor determine that Entyvio is safe and effective for you, its likely that youll use it long term.
If youre starting therapy with Entyvio, you may have questions about the medication. Here are some commonly asked questions and answers.
Intensification Of Dosing Frequency
Overall, WKM rates of dosing frequency intensification were significantly lower for the vedolizumab cohort compared with the infliximab cohort at 12 months and at 24 months after the initiation of the maintenance phase .
Weighted KaplanMeier curves of dosing frequency intensification in IBD patients . *Statistically significant at the 5% level. Dose escalation via increased dose is not included in this end point. Only dose escalation via intensification of dosing frequency is included.
WKM rates of dosing frequency intensification were significantly lower in patients with UC who received vedolizumab at 12 months and 24 months after the initiation of the maintenance phase relative to patients who received infliximab. Similar results were found in patients with CD at 12 months and at 24 months after the initiation of the maintenance phase .
Recommended Reading: Is Green Tea Good For Ulcers
Recommended Reading: Common Symptoms Of Ulcerative Colitis
Can Children And Young People Have The Vaccine
The JCVI are a group of experts that advise the government on vaccines. This group, along with Chief Medical Officers, have been looking at the benefits and effects of young people receiving the vaccine.
In England, Wales, Northern Ireland and Scotland, all children aged 12 to 15 years will be offered the Pfizer COVID-19 vaccine. The vaccine is not mandatory and parents or guardians will be asked to give consent before their child receives the vaccine.
In England, the vaccine is likely to be given as part of the school vaccination programme. For more information, visit the GOV.UK website. For young adults aged 16 to 18 years, they can attend a walk in centre for their vaccination.
In Scotland, all children and young people aged 12 to 17 years should receive an appointment letter inviting them to an appointment at a drop-in centre or vaccination clinic. For more information, visit the NHS Inform Scotland website.
In Wales, some areas will invite this age group to vaccination centres and other areas will offer the vaccine through the school vaccination programme. For more information, visit the Welsh Government website.
In Northern Ireland, the vaccine is likely to be given as part of the school vaccination programme. For more information, visit the Northern Ireland Department of Health website.Young people aged 16 to 18 years can book an appointment or find a mobile clinichere.
Key Points About Stelara
- Stelara is approved to treat Crohns disease.
- The loading dose of Stelara is given by infusion and thereafter is given by injections at home.
- People taking shots for allergies should talk to their doctor about possible allergic reactions and Stelara.
- Common side effects include infections, injection site reactions, and vomiting.
- If you are pregnant or plan to become pregnant, you and your doctor should decide if you should take Stelara.
- Its thought that Stelara does pass into breastmilk.
- Stelara must be refrigerated.
Recommended Reading: Worst Foods For Stomach Ulcers
Read Also: Do Ulcers Make You Lose Weight
Tell Your Doctor Or Ibd Team Immediately If You Develop
- Symptoms that may mean you are having a reaction to the injection or an allergic reaction:
- Hives or other skin rashes
- Trouble breathing or swallowing, or shortness of breath
- Pains in your chest or muscles or joints
- Fever or chills
- Swelling of your face, hands or feet
- Headaches or a sore throat
Living with Crohns Disease
Infliximab is often taken alongside other medicines safely. See the earlier section Taking infliximab with other Crohns or Colitis treatments.
However, infliximab may interact with other medicines. Speak to your doctor or pharmacist if youre taking, or plan to take any other medicines. This includes over the counter medicines and any herbal, complementary, or alternative medicines or therapies.
Do not take medicines that contain anakinra or abatacept. These medicines are commonly used for Rheumatoid Arthritis.
What Should I Know About Entyvio Vs Remicade
Entyvio and Remicade are both used to manage symptoms of inflammatory bowel disease. Specifically, theyre both used for Crohns disease and ulcerative colitis. Remicade is also used to manage other autoimmune conditions.
Remicade has the active ingredient infliximab and Entyvio has the active ingredient vedolizumab. Both drugs are given by intravenous infusion, which is an injection thats given slowly into your vein.
Talk with your doctor if you have more questions about Entyvio versus Remicade. You can also check out this detailed breakdown of the two medications.
Entyvio and Humira are both used to treat Crohns disease and ulcerative colitis. Humira is used to treat some other autoimmune diseases, too.
Humira has the active ingredient adalimumab, and Entyvio has the active ingredient vedolizumab.
Humira is given as an injection under the skin. You can give the drug to yourself at home.
Entyvio, on the other hand, is given at a doctors office or clinic. Its given by intravenous infusion, which is an injection thats given slowly into your vein.
If youd like to know about the similarities and differences of Entyvio and Humira, see this comparison. And talk with your doctor about which drug is right for you.
If you have a certain autoimmune disease, your doctor may prescribe Entyvio for you. Its a biologic drug that treats inflammatory bowel disease thats causing symptoms.
Specifically, Entyvio is used in adults to treat moderate to severe:
Don’t Miss: What Causes A Bleeding Ulcer In Stomach
Which Coronavirus Vaccine Will I Be Offered
The COVID-19 vaccines currently approved for use in the UK are:
- Moderna vaccine
- Pfizer/BioNTech vaccine
All of the available coronavirus vaccines are considered suitable for people with Crohns or Colitis, as they are not live vaccines. Having Crohns or Colitis, or taking medicine to treat your condition, will not affect which coronavirus vaccine is best for you.
You May Like: Ulcerative Colitis Medication Not Working
Astrazeneca Vaccine In People Under 40
The JCVI has advised that it is preferable for people under 40 to have a vaccine other than AstraZeneca. This is due to a possible, very rare, risk of blood clots The risk of blood clots is extremely rare just over 10 people develop this condition for every million doses of AstraZeneca vaccine given.
If you have concerns or are unsure about whether to have the AstraZeneca vaccine, contact your medical team for advice. When weighing up the risks and benefits, you should also consider that clotting problems are a common complication of COVID-19 infection. Deep vein thrombosis , or clotting in the legs, occurs in 11.2% of people who have COVID-19. Pulmonary embolism, or clotting on the lungs, occurs in 7.8% of people who have COVID-19.
Not everyone with Crohns or Colitis is at higher risk of severe illness from coronavirus check your risk.
Does my Crohns or Colitis increase my risk of blood clots?Crohns and Colitis are not blood clotting disorders. However, they may slightly increase your risk of blood clots. Youre more at risk during a flare-up or if youre confined to bed, for example in hospital. You can reduce your risk by not smoking, keeping active, drinking plenty of fluids, and wearing support stockings.
Read Also: Foot Ulcer Treatment At Home
The Above Policy Is Based On The Following References:
What Should I Know About Remicade Vs Stelara
Your doctor may prescribe either Stelara or Remicade if youre an adult and have any of the following conditions:
But doctors may also prescribe Stelara for some children with plaque psoriasis. Some children may also receive Remicade for ulcerative colitis and Crohns disease.
While both Remicade and Stelara can treat the same diseases, they work on different parts of the immune system.
Stelara blocks immune factors called interleukin-12 and interleukin-23. These immune factors may also play a role in certain autoimmune diseases. These are diseases that cause your immune system to attack your own body. Remicade works by blocking the activity of a type of immune system protein called tumor necrosis factor.
To learn more about these drugs and find out which might be best for you, talk with your doctor.
Read Also: What Foods Should I Eat With Ulcerative Colitis
Treatment Regimen And Follow
As per study protocol, participants received intravenous infusions of vedolizumab every 4 weeks. Patients could continue treatment until December 2016 or until withdrawal. For GEMINI 1 and C13004 patients, the first dose of open-label vedolizumab in GEMINI LTS was given no later than 9 weeks after the last dose of study drug in the prior study.
Patients could continue to receive stable doses of aminosalicylates and corticosteroids during GEMINI LTS however, at sites in the United States, patients with a clinical response to vedolizumab or who, in the opinion of the investigator, had demonstrated sufficient improvement in clinical signs and symptoms were tapered off corticosteroids using a defined regimen as required by the U.S. Food and Drug Administration for these investigational studies. Outside of the United States, corticosteroid tapering was recommended, but not required. Immunosuppressives such as azathioprine, mercaptopurine and methotrexate were permitted at stable doses at sites outside of the United States only. Topical corticosteroids were permitted during the study at all sites.
Appendix A: Examples Of Conventional Therapy Options For Cd
Mild to moderate disease induction of remission:
Mild to moderate disease maintenance of remission:
Moderate to severe disease induction of remission:
Moderate to severe disease maintenance of remission:
Perianal and fistulizing disease induction of remission:
Metronidazole ± ciprofloxacin, tacrolimus
Perianal and fistulizing disease maintenance of remission
Also Check: Whey Protein And Ulcerative Colitis
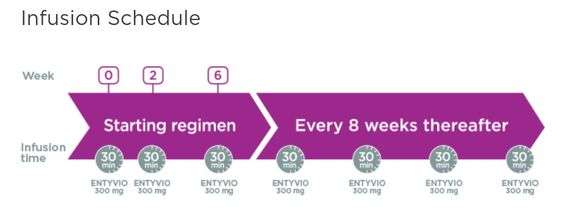
Can I Get My Dose Of Entyvio Every 4 Weeks
Its not likely. Getting a dose of Entyvio every 4 weeks isnt recommended.
Studies compared receiving Entyvio every 4 weeks with receiving it every 8 weeks for treating ulcerative colitis or Crohns disease. Researchers found that the 4-week dosing schedule didnt provide any benefit over the 8-week dosing schedule.
If you have questions about Entyvios recommended dosage, talk with your doctor.
You Are About To Leave This Website And Enter A Website Operated By An Independent Third Party
The links to third-party websites contained on this website are provided solely for your convenience. Takeda does not control the content contained on any third-party website linked from this website. Your activities at those websites will be governed by the policies and practices of those third parties.
Please select “Continue” if you wish to be taken to this third-party website.
Don’t Miss: How Much Aloe Vera Juice For Ulcers In Horses
Entyvio 108mg Solution For Injection In Pre
This information is intended for use by health professionals
Entyvio 108 mg solution for injection in pre-filled syringe
Each pre-filled syringe contains 108 mg of vedolizumab in 0.68 mL.
Vedolizumab is a humanised IgG1 monoclonal antibody produced in Chinese hamster ovary cells by recombinant DNA technology.
For the full list of excipients, see section 6.1.
Ulcerative colitis
Entyvio is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a tumour necrosis factor-alpha antagonist.
Crohn’s disease
Entyvio is indicated for the treatment of adult patients with moderately to severely active Crohn’s disease who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a tumour necrosis factor-alpha antagonist.
Treatment should be initiated and supervised by specialist healthcare professionals experienced in the diagnosis and treatment of ulcerative colitis or Crohn’s disease . Patients should be given the package leaflet.
Posology
Ulcerative colitis and Crohn’s disease
For the intravenous dose regimen, see section 4.2 of the Entyvio 300 mg powder for concentrate for solution for infusion SmPC.
There are no data on transition of patients from subcutaneous vedolizumab to intravenous vedolizumab during maintenance treatment.
Retreatment and missed dose
Special populations