The Science From Spain
In another study presented at ECCO 22 Virtual, investigators from Spain compared vedolizumab to ustekinumab after at least one anti-TNF treatment failure, this time among people with Crohns disease.
Finding effective treatments after an anti-TNF failure is essential, Maria Jose Garcia, MD, said when presenting the results of the VERSUS-CD trial. Over 20% to 30% of Crohns disease patients are nonresponders or develop intolerance to anti-TNF therapies. Also, anti-TNF responders can experience a loss of response over time.
Both vedolizumab and ustekinumab are effective for Crohns, she said. But no clinical trial has compared both treatments, and limited data exist in real life.
To remedy this situation, Garcia and colleagues studied 755 people from 30 medical centers in a national database in Spain who failed a previous anti-TNF agent, including 195 people switched to vedolizumab and 560 switched to ustekinumab. Luminal activity, perianal disease, or postoperative recurrence of Crohns were the indications for treatment.
The studys main objective was to compare the short- and long-term treatment survival rate of vedolizumab and ustekinumab after anti-TNF therapy failure in clinical practice. Evaluating efficacy and safety were secondary aims.
Just less than half of the 327 patients discontinued treatment over time, including 142 in the vedolizumab group and 185 in the ustekinumab group. The most frequent cause was primarily non-response.
What Are The Possible Side Effects And Risks Of Taking Stelara
Stelara can reduce your immune systemâs ability to fight off infections caused by viruses, fungus, or bacteria. These can be very serious or even life-threatening. Let your healthcare provider know if you develop any symptoms of infection during treatment with Stelara, such as fever, cough, weight loss, or flu-like symptoms4.
Taking Stelara can cause a slight increase in the risk of developing certain types of cancers, such as skin cancer. It can also increase the risk of a very rare condition called reversible posterior leukoencephalopathy syndrome. Healthcare providers will monitor patients for signs of any serious side effects during treatment4.
The side effects that are most frequently reported by patients who take Stelara are:
- skin reactions where the injection is delivered
This is not an exhaustive list of side effects experienced by those taking Stelara.
Nice Fails To Recommend Stelara For Ulcerative Colitis
However, draft guidance raised no concerns regarding Stelaras clinical data. Stelara is marketed for ulcerative colitis treatment in adults and is one of the companys leading pipeline assets. Stelaras failed recommendation from NICE may boost sales of Takedas Entyvio, a competitor in the ulcerative colitis market. Despite this, GlobalData forecasts that Stelaraindicated for psoriasis, ulcerative colitis, and Crohns disease in the US, EU and Japan marketswill generate global sales of $7.6B by 2025.
Stelara was not recommended as a treatment option due to uncertainty about the cost-effectiveness estimations however, Janssen has agreed to a pricing arrangement with the Commercial Medicines Unit to address public health needs. The annual cost for Stelara is £14,482 in the initial year and £9,304 per year for maintenance treatment.
Clinical trials have shown Stelara to be more effective than placebo in treating ulcerative colitis it provides a treatment option for patients not responding to conventional therapy.
You May Like: How To Treat Peptic Ulcer Pain
Key Points About Remicade
- Remicade is given by IV.
- Remicade is approved for Crohns disease and ulcerative colitis.
- Three starting doses are given .
- After the starting doses, its given about every eight weeks .
- Common side effects are abdominal pain, nausea, fatigue, and vomiting.
- If you are pregnant or plan to become pregnant, you and your doctor should decide if you should take Remicade.
Alternatives For Plaque Psoriasis
Examples of other drugs that may be used to treat plaque psoriasis include:
- topical therapies, such as oils containing vitamin D
- moderate to severe Crohns disease in adults
- moderate to severe ulcerative colitis in adults
Stelara is also approved to treat moderate to severe plaque psoriasis in children ages 6 years and older.
Humira is also approved to treat Crohns disease in children ages 6 years and older. And its approved to treat ulcerative colitis in children ages 5 years and older.
Additionally, Humira is also approved to treat:
- moderate to severe rheumatoid arthritis in adults
- uveitis in adults and children ages 2 years and older
- juvenile idiopathic arthritis in children ages 2 years and older
- hidradenitis suppurativa in adults and children ages 12 years and older
Don’t Miss: Remicade For Ulcerative Colitis Reviews
European Commission Approves Expanded Use Of Janssens Stelara For The Treatment Of Moderately To Severely Active Ulcerative Colitis In The European Union
BEERSE, Belgium—-The Janssen Pharmaceutical Companies of Johnson & Johnson announced today that the European Commission has approved the expanded use of ustekinumab for the treatment of adults with moderately to severely active ulcerative colitis , who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a biologic or have medical contraindications to such therapies.1 Ustekinumab is the first available biologic treatment to selectively target the IL-12/IL-23 pathway, an important therapeutic target in UC.2
The devastating impact of ulcerative colitis on the lives of people with this condition is often underestimated. Typically, ulcerative colitis first presents in young adults at a time when they are still in education or starting their careers often limiting their ability to achieve their personal goals, said Professor Silvio Danese*, Head of the Inflammatory Bowel Diseases Centre at Humanitas Research Hospital, Milan, Italy. Whilst there is no cure yet for ulcerative colitis, treatments which can help prevent the flare up of symptoms and allow people to get on with their lives are hugely important. For this reason, the approval of ustekinumab in ulcerative colitis is welcome news and will provide a valuable therapeutic option for both patients and their doctors.
#ENDS#
*Professor Danese is a paid consultant for Janssen. He has not been compensated for any media work.
About the UNIFI Programme
Serious Side Effects Of Stelara
Rarely, Stelara may cause serious side effects. Serious side effects werent common in clinical trials. The list below may not include all possible reported serious side effects of the drug. For more information, you can refer to the Stelara medication guide.
If you develop serious side effects while taking Stelara, call your doctor right away. If the side effects seem life threatening, or if you think youre having a medical emergency, immediately call 911 or your local emergency number.
Serious side effects and their symptoms can include:
- reversible posterior leukoencephalopathy syndrome
Stelara may cause several side effects. Here are some frequently asked questions about the drugs side effects and their answers.
Recommended Reading: Natural Treatment Of Ulcer In Hindi
You May Like: Icd 10 Venous Stasis Ulcer Left Leg
Pregnancy And Breastfeeding While Taking Stelara
It isnt known whether Stelara is safe to take while either pregnant or breastfeeding. Studies in pregnant animals didnt show harm to the fetus when Stelara was given to pregnant females. Animal studies also showed that, when Stelara was given to lactating females, the drug passed into breast milk.
However, its important to remember that animal studies dont always predict what will happen in people. There isnt enough information to know for sure how the drug may affect human pregnancy or breastfeeding.
If you have additional questions about using Stelara while pregnant or breastfeeding, talk with your doctor.
If you took Stelara during a past pregnancy or if youre currently taking it during pregnancy, youre encouraged join a pregnancy registry. The goal of this registry is to help doctors and people taking the drug learn more about the drugs safety. Consider calling the registry at 866-626-6847. Or you can visit the program website.
Stelara is approved to treat the following in certain conditions:
- moderate-to-severe plaque psoriasis in adults, as well as children ages 6 years and older
- moderate-to-severe ulcerative colitis in adults
Regardless of the condition its used to treat, side effects from Stelara arent common. Most side effects that occur are usually mild, and tend to go away on their own.
Rarely, Stelara may cause some serious side effects. Contact your doctor if you experience:
Get emergency medical help right away if you experience:
Dosage For Plaque Psoriasis
Stelara is approved to treat plaque psoriasis in both adults and children ages 6 years and older. The typical dosage for adults is described here.
Stelara is given as one subcutaneous injection on each of the following days:
- your first dose is given on day 1
- your second dose is given 4 weeks later
- your third dose is given 12 weeks after your second dose
- the rest of your doses are given every 12 weeks
The usual dosage of Stelara for plaque psoriasis is based on your body weight and age. In adults with plaque psoriasis, the typical dosage of Stelara for each injection is as follows:
- for adults who weigh 100 kilograms or less, their usual dosage is 45 mg
- for adults who weigh more than 100 kilograms, their usual dosage is 90 mg
Don’t Miss: Indian Diet For Ulcerative Colitis
Fda Approves Ustekinumab For Patients With Acute Ulcerative Colitis
Ustekinumab both induced and maintained clinical remission in a significantly greater proportion of adult patients with moderately to severely active ulcerative colitis compared with placebo.
The FDA has approved ustekinumab for the treatment of adult patients with moderately to severely active ulcerative colitis .
The approval is based on the pivotal phase 3 UNIFI clinical trial, which demonstrated that treatment with ustekinumab both induced and maintained clinical remission in a significantly greater proportion of adult patients with moderately to severely active UC compared with placebo.
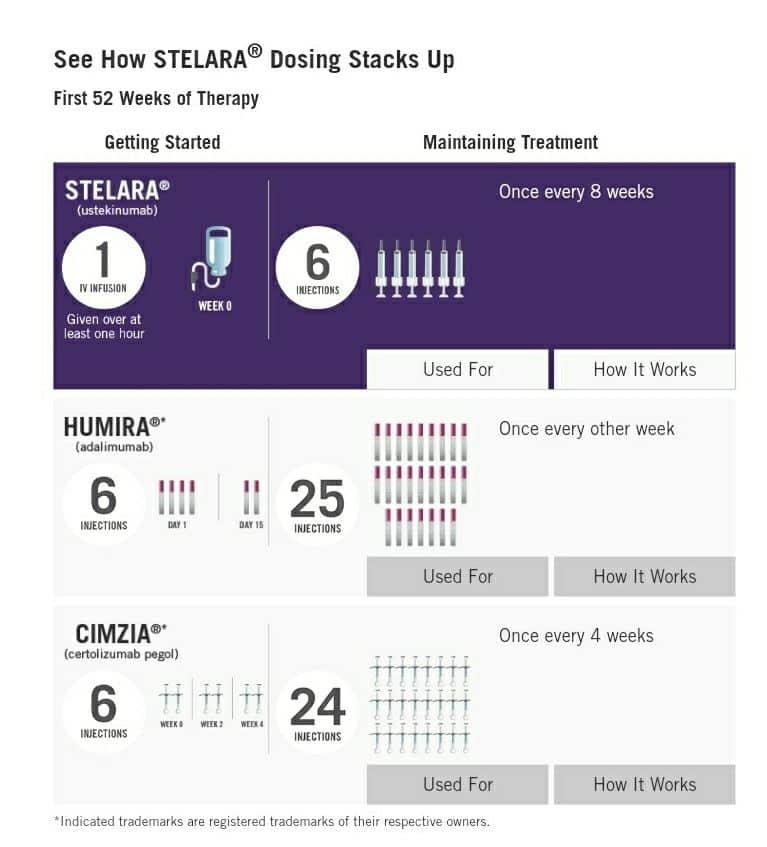
Published in the New England Journal of Medicine, the pivotal trial included an initial induction study in which patients received a single dose of ustekinumab 6 mg/kg intravenous infusion. It was followed 8 weeks later by a Maintenance study , in which patients received ustekinumab 90 mg subcutaneous injections every 8 weeks for 44 weeks.
In the induction study, 19% of patients receiving ustekinumab achieved clinical remission in 8 weeks and the drug therapy provided rapid relief of symptoms, as 58% of patients receiving ustekinumab experienced a clinical response at week 8.
In the maintenance study, 45% of patients receiving ustekinumab were in remission at 1 year. Ustekinumab also helped patients achieve clinical remission without the use of corticosteroids. At 1 year, 43% of patients treated with ustekinumab were in clinical remission and not receiving steroids.
Reference
Ustekinumab Receives Fda Approval For Ulcerative Colitis
Ustekinumab has received approval from the US Food and Drug Administration for the treatment of moderate to severely active ulcerative colitis in adults.
Adult patients with moderate to severely active ulcerative colitis have a new treatment option with the United States Food and Drug Administrations approval of ustekinumab.
With the approval, which was awarded to Janssen Pharmaceutical, ustekinumab becomes the first and only approved treatment for ulcerative colitis to demonstrate improvement of the colon.
The FDA approval of Stelara for UC represents an exciting milestone, offering patients a new option that has demonstrated improvement of the histology and endoscopic appearance of the intestinal lining, while also offering patients the potential for response and remission without the need for steroids, said William Sandborn, MD, professor of medicine UC San Diego School of Medicine and clinical trial investigator.
Approval of ustekinumab was based on results of the phase 3 UNIFI clinical trial, which demonstrated the therapys ability to induce remission in more than 40% of patients at 1 year. The trial had a primary endpoint of clinical remission
Investigators observed 19% of patients receiving ustekinumab achieved clinical remission during the induction study. Investigators also noted ustekinumab provided rapid relief of symptoms in 58% of patients during the 8-week study.
Related Content:
Recommended Reading: Food For Ulcer Patient In Nigeria
General Information About The Safe And Effective Use Of Stelara
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Stelara for a condition for which it was not prescribed. Do not give Stelara to other people, even if they have the same symptoms that you have. It may harm them. You can ask your doctor or pharmacist for information about Stelara that was written for health professionals.
Will I Need To Use This Drug Long Term
Stelara is meant to be used as a long-term treatment. If you and your doctor determine that Stelara is safe and effective for you, youll likely take it long term.
As with all medications, the cost of Stelara can vary. The actual price youll pay depends on your insurance plan, your location, and the pharmacy you use.
Recommended Reading: How Can You Tell If Your Horse Has Ulcers
Stelara For Crohns Disease
Stelara is FDA-approved to treat moderate to severe Crohns disease in adults.
Crohns disease is a type of inflammatory bowel disease. It causes inflammation in your gastrointestinal tract.
With Crohns disease, you may have:
- pain and cramps in your belly
Effectiveness for Crohns disease
One clinical study looked at effectiveness after 8 weeks of treatment. In this study, clinical remission was reached in 14% to 21% more people who took Stelara than in those who took a placebo .
Clinical remission can also be defined based on a scoring system that measures how severe peoples Crohns disease symptoms are. Lower scores show fewer Crohns disease symptoms. If you have a score of less than 150 points, youre considered to be in remission. The highest possible score is 1,100.
In these 8-week studies, between 18% and 26% more people taking Stelara than those taking the placebo had a decreased score of at least 100 points.
Stelara And Other Medications Or Therapies
Below are lists of medications and therapies that can interact with Stelara. These lists do not contain all the drugs that may interact with Stelara.
Before taking Stelara, talk with your doctor and pharmacist. Tell them about all prescription, over-the-counter, and other drugs you take. Also tell them about any vitamins, herbs, and supplements you use. Sharing this information can help you avoid potential interactions.
If you have questions about drug interactions that may affect you, ask your doctor or pharmacist.
Stelara and vaccines
You shouldnt get a live vaccine when youre using Stelara. Getting a live vaccine during Stelara treatment increases your risk of getting the condition the vaccine is meant to prevent.
This is because Stelara suppresses your immune systems ability to fight infections. Receiving a live vaccine during Stelara treatment increases your risk of serious infections.
Examples of live vaccines that you should avoid during Stelara treatment include:
You should also avoid getting the Bacillus Calmette-Guérin vaccine for 1 year before you start using Stelara, during your Stelara treatment, and for 1 year after you stop using Stelara. The BCG vaccine is meant to prevent tuberculosis . Its more commonly given to people who live outside of the United States.
Stelara and allergy shots
Its not known if its safe to get allergy shots while youre using Stelara. This is because Stelara may affect how your immune system responds to the allergy shots.
Recommended Reading: Is Keto Good For Ulcerative Colitis
Financial And Insurance Assistance
If you need financial support to pay for Stelara, or if you need help understanding your insurance coverage, help is available.
Janssen Biotech, Inc., the manufacturer of Stelara, offers a program called Janssen CarePath. For more information and to find out if youre eligible for support, call 877-CAREPATH or visit the program website.
Your doctor will recommend if you need to take other drugs with Stelara to treat your condition.
Report A Janssen Product Adverse Event
You may contact the Medical Information Center by calling 1-800-JANSSEN to speak to a clinical expert regarding your question or to report a possible adverse event.
Report a Janssen COVID-19 Vaccine Adverse Event
Report a Adverse Event to FDA VAERS at www.vaers.hhs.gov/reportevent.html or call the FDA at 1-800-822-7967.Report a Adverse Event to Janssen at 1-800-565-4008 or 1-908-455-8822 .
Read Also: What Is Stomach Ulcer Pain Like
How Is Stelara Given
- In ulcerative colitis in adults, Stelara is initially given as a one-time, weight-based intravenous infusion dose.
- It is followed by a maintenance injection dose of 90 mg given by a subcutaneous injection every 8 weeks.
In studies, most patients saw rapid relief from UC symptoms in 8 weeks, with 20% of patients reaching remission. At one year, 40% of patients were in remission. At 2 years, 70% of patients had no rectal bleeding and fewer daily bowel movements.
The most common side effects in ulcerative colitis studies include:
- Nasopharyngitis
What Are The Forms Of Stelara
Stelara comes as a liquid solution in either in a single-dose prefilled syringe or in a single-dose vial. The drug can be given in two ways: as a subcutaneous injection or as an intravenous infusion.
If you receive Stelara by IV infusion, youll get your doses from a healthcare professional, such as at your doctors office or a clinic. Its also recommended that children receiving subcutaneous injections of Stelara get their doses in the doctors office or a clinic.
Adults using the subcutaneous injection form of Stelara may want to learn how to inject the drug themselves or have a caregiver administer their doses of Stelara. If your doctor determines that this is an option for you, theyll give you dosing instructions.
If youre interested in using Stelara at home, talk with your doctor.
Recommended Reading: Preventing Pressure Ulcers In Nursing Homes
Don’t Miss: Pressure Relieving Mattress For Pressure Ulcers
Can I Take Ustekinumab With Other Medication
Most other medications can be taken safely whilst on Stelara. Your doctor will advise you if it is safe to take other medications at the same time as Stelara. Its important that you provide a full list of the medications, including non-prescribed, you are taking, or have taken recently, especially if those medications affect your immune system.
Some patients will be prescribed other medications alongside ustekinumab, such as azathioprine, methotrexate and steroids.